有机化学 ›› 2022, Vol. 42 ›› Issue (12): 4292-4299.DOI: 10.6023/cjoc202211002 上一篇 下一篇
所属专题: 自由基化学专辑
研究论文
穆思宇a, 李红霞a, 伍智林b, 彭俊梅a,*( ), 陈锦杨b, 何卫民b,*(
), 陈锦杨b, 何卫民b,*( )
)
收稿日期:2022-11-01
修回日期:2022-11-20
发布日期:2022-11-28
通讯作者:
彭俊梅, 何卫民
基金资助:
Siyu Mua, Hongxia Lia, Zhilin Wub, Junmei Penga( ), Jinyang Chenb, Weimin Heb(
), Jinyang Chenb, Weimin Heb( )
)
Received:2022-11-01
Revised:2022-11-20
Published:2022-11-28
Contact:
Junmei Peng, Weimin He
Supported by:文章分享

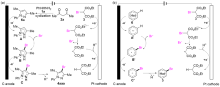
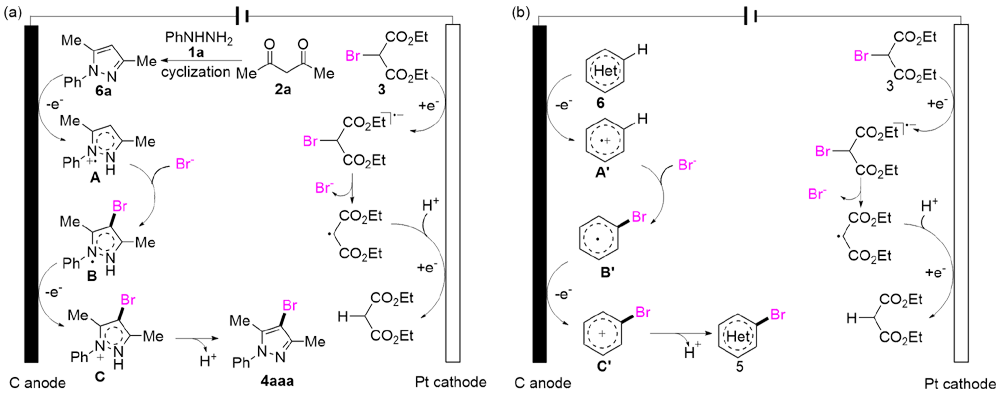
吡唑是一类重要的五元氮杂环化合物, 存在于多种天然产物和药物分子中. 4-溴吡唑是一种高价值的吡唑衍生物, 不仅具有多种生物活性和药理活性, 还被广泛用作有机合成中间体, 因此发展新型高效的4-溴吡唑化合物合成方法具有重要的研究意义. 本研究发展了一种电化学促进芳香肼、1,2-丙二酮和2-溴丙二酸二乙酯三组分串联环化-溴化反应合成4-溴吡唑化合物的方法, 并揭示了其反应机理. 反应历程为芳香肼与丙二酮发生环化反应得到吡唑杂环化合物, 其发生溴化反应得到4-溴吡唑. 2-溴丙二酸二乙酯具有较高的氧化态, 防止电解过程中过氧化效应, 避免了过量溴化剂的使用. 我们还将该方法用于其他溴化杂环化合物的合成.
穆思宇, 李红霞, 伍智林, 彭俊梅, 陈锦杨, 何卫民. 电催化肼、丙二酮和2-溴丙二酸二乙酯三组分合成4-溴吡唑[J]. 有机化学, 2022, 42(12): 4292-4299.
Siyu Mu, Hongxia Li, Zhilin Wu, Junmei Peng, Jinyang Chen, Weimin He. Electrocatalytic Three-Component Synthesis of 4-Bromopyrazoles from Acetylacetone, Hydrazine and Diethyl Bromomalonate[J]. Chinese Journal of Organic Chemistry, 2022, 42(12): 4292-4299.
| Entry | Variation from the standard reaction conditions | Yieldb/% |
|---|---|---|
| 1 | None | 96 |
| 2 | Pt(+)|Cu(–) instead of C(+)|Pt (–) | 86 |
| 3 | Pt (+)|Ni(–) instead of C(+)|Pt (–) | 90 |
| 4 | Pt (+)|Pt(–) instead of C(+)|Pt (–) | 92 |
| 5 | Pt (+)|Al(–) instead of C(+)|Pt (–) | 86 |
| 6 | C(+)|C(–) instead of C(+)|Pt (–) | 87 |
| 7 | C(+)|Ni(–) instead of C(+)|Pt (–) | 88 |
| 8 | Pt(+)|SS(–) instead of C(+)|Pt (–) | 84 |
| 10 | 1.0 equiv. of Et4NBF4 instead of nBu4N- BF4 | 92 |
| 11 | 1.0 equiv. of KPF4 instead of nBu4NBF4 | 91 |
| 12 | Acetone instead of MeCN | 85 |
| 13 | EtOH instead of MeCN | 82 |
| 14 | DMF instead of MeCN | 92 |
| 15 | DMSO instead of MeCN | 90 |
| 16 | 1.0 equiv. of NaBr instead of 3 | 75 |
| 17 | 2.0 equiv. of NaBr instead of 3 | 88 |
| 18 | 3.0 equiv. of NaBr instead of 3 | 92 |
| 19 | 2.0 equiv. of KBr instead of 3 | 87 |
| 20 | 2.0 equiv. of nBu4NBr instead of 3 | 88 |
| 21 | 5 mA, 15 h instead of 8 mA, 10 h | 82 |
| 22 | 15 mA, 4 h instead of 8 mA, 10 h | 83 |
| 23 | Without electric current | N.R.c |
| Entry | Variation from the standard reaction conditions | Yieldb/% |
|---|---|---|
| 1 | None | 96 |
| 2 | Pt(+)|Cu(–) instead of C(+)|Pt (–) | 86 |
| 3 | Pt (+)|Ni(–) instead of C(+)|Pt (–) | 90 |
| 4 | Pt (+)|Pt(–) instead of C(+)|Pt (–) | 92 |
| 5 | Pt (+)|Al(–) instead of C(+)|Pt (–) | 86 |
| 6 | C(+)|C(–) instead of C(+)|Pt (–) | 87 |
| 7 | C(+)|Ni(–) instead of C(+)|Pt (–) | 88 |
| 8 | Pt(+)|SS(–) instead of C(+)|Pt (–) | 84 |
| 10 | 1.0 equiv. of Et4NBF4 instead of nBu4N- BF4 | 92 |
| 11 | 1.0 equiv. of KPF4 instead of nBu4NBF4 | 91 |
| 12 | Acetone instead of MeCN | 85 |
| 13 | EtOH instead of MeCN | 82 |
| 14 | DMF instead of MeCN | 92 |
| 15 | DMSO instead of MeCN | 90 |
| 16 | 1.0 equiv. of NaBr instead of 3 | 75 |
| 17 | 2.0 equiv. of NaBr instead of 3 | 88 |
| 18 | 3.0 equiv. of NaBr instead of 3 | 92 |
| 19 | 2.0 equiv. of KBr instead of 3 | 87 |
| 20 | 2.0 equiv. of nBu4NBr instead of 3 | 88 |
| 21 | 5 mA, 15 h instead of 8 mA, 10 h | 82 |
| 22 | 15 mA, 4 h instead of 8 mA, 10 h | 83 |
| 23 | Without electric current | N.R.c |
| [1] |
Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y. N.; Al-aizari, F. A.; Ansar, M. Molecules 2018, 23, 134.
doi: 10.3390/molecules23010134 |
| [2] |
Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman New J. Chem. 2017, 41, 16.
doi: 10.1039/C6NJ03181A |
| [3] |
Sun, K.; Xiao, F.; Yu, B.; He, W.-M. Chin. J. Catal. 2021, 42, 1921.
doi: 10.1016/S1872-2067(21)63850-0 |
| [4] |
Bennani, E. F.; Doudach, L.; Cherrah, Y.; Ramli, Y.; Karrouchi, K.; Ansar, M.; Faouzi, M. E. A. Bioorg. Chem. 2020, 97, 103470.
doi: 10.1016/j.bioorg.2019.103470 |
| [5] |
Bekhit, A. A.; Ashour, H. M. A.; Guemei, A. A. Arch Pharm. 2005, 338, 167.
pmid: 15864786 |
| [6] |
Jiang, J.; Wang, Z.; He, W.-M. Chin. Chem. Lett. 2021, 32, 1591.
doi: 10.1016/j.cclet.2021.02.067 |
| [7] |
Zehnder, L.; Bennett, M.; Meng, J.; Huang, B.; Ninkovic, S.; Wang, F.; Braganza, J.; Tatlock, J.; Jewell, T.; Zhou, J. Z.; Burke, B.; Wang, J.; Maegley, K.; Mehta, P. P.; Yin, M.-J.; Gajiwala, K. S.; Hickey, M. J.; Yamazaki, S.; Smith, E.; Kang, P.; Sistla, A.; Dovalsantos, E.; Gehring, M. R.; Kania, R.; Wythes, M.; Kung, P.-P. J. Med. Chem. 2011, 54, 3368.
doi: 10.1021/jm200128m pmid: 21438541 |
| [8] |
Kaur, G.; Utreja, D.; Jain, N.; Dhillon, N. K. Russ. J. Org. Chem. 2020, 56, 113.
doi: 10.1134/S1070428020010182 |
| [9] |
Wood, D. J.; Lopez-Fernandez, J. D.; Knight, L. E.; Al-Khawaldeh, I.; Gai, C.; Lin, S.; Martin, M. P.; Miller, D. C.; Cano, C.; Endicott, J. A.; Hardcastle, I. R.; Noble, M. E. M.; Waring, M. J. J. Med. Chem. 2019, 62, 3741.
doi: 10.1021/acs.jmedchem.9b00304 |
| [10] |
Ichikawa, H.; Ohno, Y.; Usami, Y.; Arimoto, M. Heterocycles 2006, 68, 2247.
doi: 10.3987/COM-06-10863 |
| [11] |
Balle, T.; Andersen, K.; Vedso, P. Synthesis 2002, 1509.
|
| [12] |
Willy, B.; Muller, T. J. J. Org. Lett. 2011, 13, 2082.
doi: 10.1021/ol2004947 |
| [13] |
Stefani, H. A.; Pereira, C. M. P.; Almeida, R. B.; Braga, R. C.; Guzen, K. P.; Cella, R. Tetrahedron Lett. 2005, 46, 6833.
doi: 10.1016/j.tetlet.2005.08.027 |
| [14] |
Kakarla, G. Li, R.; Gerritz, S. W. Tetrahedron Lett. 2007, 48, 4595.
doi: 10.1016/j.tetlet.2007.04.118 |
| [15] |
He, J.; Wei, Y.; Feng, Y.; Li, C.; Dai, B.; Liu, P. Synthesis 2021, 54, 1793.
doi: 10.1055/a-1684-0308 |
| [16] |
Olsen, K. L.; Jensen, M. R.; MacKay, J. A. Tetrahedron Lett. 2017, 58, 4111.
doi: 10.1016/j.tetlet.2017.09.042 |
| [17] |
Ichikawa, H.; Nishioka, M.; Arimoto, M.; Usami, Y. Heterocycles 2010, 81, 1509.
doi: 10.3987/COM-10-11954 |
| [18] |
Petzold, D.; König, B. Adv. Synth. Catal. 2018, 360, 626.
doi: 10.1002/adsc.201701276 |
| [19] |
Yuan, Y.; Yao, A.; Zheng, Y.; Gao, M.; Zhou, Z.; Qiao, J.; Hu, J.; Ye, B.; Zhao, J.; Wen, H.; Lei, A. iScience 2019, 12, 293.
doi: 10.1016/j.isci.2019.01.017 |
| [20] |
Chen, J.-Y.; Wu, H.-Y.; Gui, Q.-W.; Yan, S.-S.; Deng, J.; Lin, Y.-W.; Cao, Z.; He, W.-M. Chin. J. Catal. 2021, 42, 1445.
doi: 10.1016/S1872-2067(20)63750-0 |
| [21] |
Liang, Y.; Lin, F.; Adeli, Y.; Jin, R.; Jiao, N. Angew. Chem., Int. Ed. 2019, 58, 4566.
doi: 10.1002/anie.201814570 |
| [22] |
Wu, Y.; Chen, J.-Y.; Liao, H.-R.; Shu, X.-R.; Duan, L.-L.; Yang, X.-F.; He, W.-M. Green Synth. Catal. 2021, 2, 233.
|
| [23] |
Kingston, C.; Palkowitz, M. D.; Takahira, Y.; Vantourout, J. C.; Peters, B. K.; Kawamata, Y.; Baran, P. S. Acc. Chem. Res. 2020, 53, 72.
doi: 10.1021/acs.accounts.9b00539 |
| [24] |
Chen, N.; Xu, H.-C., Green Synth. Catal. 2021, 2, 165.
|
| [25] |
Yang, Z.; Yu, Y.; Lai, L.; Zhou, L.; Ye, K.; Chen, F.-E. Green Synth. Catal. 2021, 2, 19.
|
| [26] |
Jiang, B.; Rajale, T.; Wever, W.; Tu, S.-J.; Li, G. Chem.-Asian J. 2010, 5, 2318.
doi: 10.1002/asia.201000310 |
| [27] |
Dömling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083.
doi: 10.1021/cr100233r |
| [28] |
Meng, N.; Lv, Y.; Liu, Q.; Liu, R.; Zhao, X.; Wei, W. Chin. Chem. Lett. 2021, 32, 258.
doi: 10.1016/j.cclet.2020.11.034 |
| [29] |
Ma, C.-H.; Ji, Y.; Zhao, J.; He, X.; Zhang, S.-T.; Jiang, Y.-Q.; Yu, B. Chin. J. Catal. 2022, 43, 571.
doi: 10.1016/S1872-2067(21)63917-7 |
| [30] |
Cao, M.; Fang, Y.-L.; Wang, Y.-C.; Xu, X.-J.; Xi, Z.-W.; Tang, S. ACS Comb. Sci. 2020, 22, 268.
doi: 10.1021/acscombsci.0c00012 |
| [31] |
Cao, M.; Liu, L.; Tang, S.; Peng, Z.; Wang, Y. Adv. Synth. Catal. 2019, 361, 1887.
doi: 10.1002/adsc.201801245 |
| [32] |
Yang, Q.-L.; Ma, R.-C.; Li, Z.-H.; Li, W.-W.; Qu, G.-R.; Guo, H.-M. Org. Chem. Front. 2022, 9, 4990.
doi: 10.1039/D2QO00904H |
| [33] |
Wang, Z.-H.; Wei, L.; Jiao, K.-J.; Ma, C.; Mei, T.-S. Chem. Commun. 2022, 58, 8202.
doi: 10.1039/D2CC02641D |
| [34] |
Zhong, P.-F.; Lin, H.-M.; Wang, L.-W.; Mo, Z.-Y.; Meng, X.-J.; Tang, H.-T.; Pan, Y.-M. Green Chem. 2020, 22, 6334.
doi: 10.1039/D0GC02125C |
| [35] |
He, M.-X.; Mo, Z.-Y.; Wang, Z.-Q.; Cheng, S.-Y.; Xie, R.-R.; Tang, H.-T.; Pan, Y.-M. Org. Lett. 2020, 22, 724.
doi: 10.1021/acs.orglett.9b04549 |
| [36] |
Li, Q.-Y.; Cheng, S.-Y.; Tang, H.-T.; Pan, Y.-M. Green Chem. 2019, 21, 5517.
doi: 10.1039/C9GC03028J |
| [37] |
Chen, J.-Y.; Li, H.-X.; Mu, S.-Y.; Song, H.-Y.; Wu, Z.-L.; Yang, T.-B.; Jiang, J.; He, W.-M. Org. Biomol. Chem. 2022, 20, 8501.
doi: 10.1039/D2OB01612E |
| [38] |
Wu, Z.-L.; Chen, J.-Y.; Tian, X.-Z.; Ouyang, W.-T.; Zhang, Z.-T.; He, W.-M. Chin. Chem. Lett. 2022, 33, 1501.
doi: 10.1016/j.cclet.2021.08.071 |
| [39] |
Chen, J.-Y.; Zhong, C.-T.; Gui, Q.-W.; Zhou, Y.-M.; Fang, Y.-Y.; Liu, K.-J.; Lin, Y.-W.; Cao, Z.; He, W.-M. Chin. Chem. Lett. 2021, 32, 475.
doi: 10.1016/j.cclet.2020.09.034 |
| [40] |
Wu, Y.; Chen, J.-Y. Ning, J.; Jiang, X.; Deng, J.; Deng, Y.; Xu, R.; He, W.-M. Green Chem. 2021, 23, 3950.
doi: 10.1039/D1GC00562F |
| [41] |
Gui, Q.-W. G.; Teng, F.; Yu, P.; Wu, Y.-F.; Nong, Z.-B.; Yang, L.-X. Y.; Chen, X.; Yang, T.-B.; He, W.-M. Chin. J. Catal. 2023, 44, 111
doi: 10.1016/S1872-2067(22)64162-7 |
| [42] |
Zhang, T.-T. Luo, M.-J.; Li, Y.; Song, R.-J.; Li, J.-H. Org. Lett. 2020, 22, 7250.
doi: 10.1021/acs.orglett.0c02582 |
| [43] |
Bondarenko, O. B.; Karetnikov, G. L.; Komarov, A. I.; Pavlov, A. I.; Nikolaeva, S. N. J. Org. Chem. 2021, 86, 322.
doi: 10.1021/acs.joc.0c02106 pmid: 33347755 |
| [44] |
Hanthorn, J. J.; Valgimigli, L.; Pratt, D. A. J. Org. Chem. 2012, 77, 6908.
doi: 10.1021/jo301013c pmid: 22788575 |
| [45] |
Schwertz, G.; Witschel, M. C.; Rottmann, M.; Bonnert, R.; Leartsakulpanich, U.; Chitnumsub, P.; Jaruwat, A.; Ittarat, W.; Schäfer, A.; Aponte, R. A.; Charman, S. A.; White, K. L.; Kundu, A.; Sadhukhan, S.; Lloyd, M.; Freiberg, G. M.; Srikumaran, M.; Siggel, M.; Zwyssig, A.; Chaiyen, P.; Diederich, F. J. Med. Chem. 2017, 60, 4840.
doi: 10.1021/acs.jmedchem.7b00008 |
| [46] |
Shang, X.; Liu, X.; Sun, Y. Green Chem. 2021, 23, 2037.
doi: 10.1039/D0GC04362A |
| [47] |
Das, D.; Samanta, R. Adv. Synth. Catal. 2018, 360, 379.
doi: 10.1002/adsc.201701244 |
| [1] | 钟赟哲, 陈颖, 俞磊, 周宏伟. 电化学介导羧酸与醇的酯化反应[J]. 有机化学, 2023, 43(8): 2855-2863. |
| [2] | 高师泉, 刘闯军, 杨俊锋, 张俊良. 钴催化的烯烃和炔烃的电化学还原偶联反应[J]. 有机化学, 2023, 43(4): 1559-1565. |
| [3] | 张玉荣, 王晗, 茆勇军, 施世良. 镍催化丁二烯、亚胺和烯基硼酸的三组分偶联反应[J]. 有机化学, 2022, 42(4): 1198-1209. |
| [4] | 易荣楠, 刘冬娴, 吴啟林, 赵明明, 王勇, 王峥. 电化学氧化-碘促进丙酮α-H芳(烷)硒化制备α-芳(烷)硒基丙酮[J]. 有机化学, 2021, 41(9): 3726-3732. |
| [5] | 马蔚青, 韩莹, 孙晶, 颜朝国. 三组分反应高效合成螺[环戊烷-1,3'-吲哚啉]衍生物[J]. 有机化学, 2021, 41(8): 3180-3191. |
| [6] | 孟薇, 徐坤, 郭兵兵, 曾程初. 电化学条件下的Minisci反应研究进展[J]. 有机化学, 2021, 41(7): 2621-2635. |
| [7] | 何慕雪, 程诗砚, 潘永周, 唐海涛, 潘英明. 电化学介导的S—N键形成: 次磺酰胺化合物的简洁合成[J]. 有机化学, 2021, 41(6): 2354-2360. |
| [8] | 李雨青, 施世良. 镍催化的丁二烯、醛、炔和氢氯二茂锆的多组分偶联反应合成1,4-二烯[J]. 有机化学, 2021, 41(5): 1939-1948. |
| [9] | 殷国栋, 李源, 范玲. 四氯化锆催化合成嘧啶并[4,5-b]喹啉-2,4(1H,3H)-二酮和11H-茚[1,2-b]喹啉-11-酮[J]. 有机化学, 2021, 41(3): 1234-1240. |
| [10] | 王琦, 朱柏燃, 杨光, 马献涛, 徐清. 无碱条件下直接多组分反应选择性合成非对称含氮杂芳基硫醚[J]. 有机化学, 2021, 41(3): 1193-1199. |
| [11] | 闫强, 范荣, 刘斌斌, 苏帅松, 王勃, 姚团利, 谭嘉靖. 苯炔参与的去芳构化反应研究进展[J]. 有机化学, 2021, 41(2): 455-470. |
| [12] | 程诗砚, 欧楚鸿, 林洪敏, 贾均松, 唐海涛, 潘英明, 黄国保, 蒙秀金. 电化学介导的芳香醛和脂肪醇氧化酯化反应[J]. 有机化学, 2021, 41(12): 4718-4724. |
| [13] | 秦锋, 汤琳, 黄飞, 李晓悦, 张武. 铜催化氧化和Aza-Diels-Alder反应三组分合成喹啉[J]. 有机化学, 2021, 41(1): 318-324. |
| [14] | 肖立伟, 刘光仙, 李政, 任萍, 任丽磊, 孔洁. 低共熔溶剂促进N-取代十氢吖啶-1,8-二酮类化合物的合成[J]. 有机化学, 2020, 40(9): 2988-2993. |
| [15] | 孙晓华, 孙传策, 冯立军, 康从民. 串联环化反应合成噻吩并[2,3-d]嘧啶类化合物的研究进展[J]. 有机化学, 2020, 40(9): 2626-2640. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||