有机化学 ›› 2019, Vol. 39 ›› Issue (11): 3176-3182.DOI: 10.6023/cjoc201905017 上一篇 下一篇
研究论文
收稿日期:2019-05-09
发布日期:2019-07-09
通讯作者:
陈晓培
E-mail:chenxp@hnuahe.edu.cn
基金资助:
Chen Xiaopei*( ), Ma Zhiwei, Wang Chuanchuan, Liu Juntao, Wu Jinsong
), Ma Zhiwei, Wang Chuanchuan, Liu Juntao, Wu Jinsong
Received:2019-05-09
Published:2019-07-09
Contact:
Chen Xiaopei
E-mail:chenxp@hnuahe.edu.cn
Supported by:文章分享

以醇为酰基化试剂,在水溶液条件下,发展了一种简便、高效的钯催化氧化偶氮苯酰基化反应体系.在此体系中,醇被氧化为醛,实现氧化偶氮苯的邻位酰基化反应,具有很好的区域选择性,以中等到较高收率得到酰基化的氧化偶氮苯衍生物,底物的普适性较好.
中图分类号:
陈晓培, 马志伟, 王川川, 刘俊桃, 吴金松. 在水相中钯催化氧化偶氮苯的区域选择性邻位酰基化反应[J]. 有机化学, 2019, 39(11): 3176-3182.
Chen Xiaopei, Ma Zhiwei, Wang Chuanchuan, Liu Juntao, Wu Jinsong. Palladium-Catalyzed Regioselective ortho-Acylation of Azoxybenzenes under Aqueous Conditions[J]. Chinese Journal of Organic Chemistry, 2019, 39(11): 3176-3182.
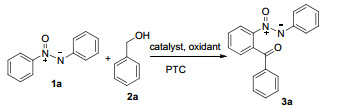 | ||||
| Entrya | Catalyst | Oxidant | PTC | Yield b/% |
| 1 | PdCl2 | TBHP | 15 | |
| 2 | PdCl2 | TBHP | SDS | 35 |
| 3 | Pd(TFA)2 | TBHP | SDS | 68 |
| 4 | Pd(OAc)2 | TBHP | SDS | 80 |
| 5 | Pd(OAc)2 | DBHP | SDS | 20 |
| 6 | Pd(OAc)2 | AgOAc | SDS | 15 |
| 7 | Pd(OAc)2 | K2S2O8 | SDS | Trace |
| 8 | Pd(OAc)2 | (NH4)2S2O8 | SDS | Trace |
| 9 | Pd(OAc)2 | BQ | SDS | NDe |
| 10 | Pd(OAc)2 | DDQ | SDS | NDe |
| 11 | Pd(OAc)2 | H2O2 | SDS | NDe |
| 12 | Pd(OAc)2 | TBHP | TBAB | 20 |
| 13 | Pd(OAc)2 | TBHP | 18-Crown-6 | 42 |
| 14 | Pd(OAc)2 | TBHP | Tween 80 | 10 |
| 15c | Pd(OAc)2 | TBHP | SDS | 65 |
| 16d | Pd(OAc)2 | TBHP | SDS | 75 |
 | ||||
| Entrya | Catalyst | Oxidant | PTC | Yield b/% |
| 1 | PdCl2 | TBHP | 15 | |
| 2 | PdCl2 | TBHP | SDS | 35 |
| 3 | Pd(TFA)2 | TBHP | SDS | 68 |
| 4 | Pd(OAc)2 | TBHP | SDS | 80 |
| 5 | Pd(OAc)2 | DBHP | SDS | 20 |
| 6 | Pd(OAc)2 | AgOAc | SDS | 15 |
| 7 | Pd(OAc)2 | K2S2O8 | SDS | Trace |
| 8 | Pd(OAc)2 | (NH4)2S2O8 | SDS | Trace |
| 9 | Pd(OAc)2 | BQ | SDS | NDe |
| 10 | Pd(OAc)2 | DDQ | SDS | NDe |
| 11 | Pd(OAc)2 | H2O2 | SDS | NDe |
| 12 | Pd(OAc)2 | TBHP | TBAB | 20 |
| 13 | Pd(OAc)2 | TBHP | 18-Crown-6 | 42 |
| 14 | Pd(OAc)2 | TBHP | Tween 80 | 10 |
| 15c | Pd(OAc)2 | TBHP | SDS | 65 |
| 16d | Pd(OAc)2 | TBHP | SDS | 75 |
| [1] |
(a) Surburg, H.; Panten, J. Common Fragrance and Flavour Materials, Wiley Online Library, 2006.
doi: 10.1021/ed1003806 |
|
(b) McGrath, N. A.; Brichacek, M.; Njardarson, J. T. J. Chem. Educ. 2010, 87, 1348.
doi: 10.1021/ed1003806 |
|
| [2] |
(a) Olah, G. A. Friedel-Crafts Chemistry, Wiley, New York, 1973.
doi: 10.1021/cr040695c |
|
(b) Sartori, G.; Maggi, R. Chem. Rev. 2006, 106, 1077.
doi: 10.1021/cr040695c |
|
|
(c) Fernandez, M.; Tojo, G. In Oxidation of Alcohols to Aldehydes and Ketones: A Guideto Current Common Practice, Ed.: Tojo, E., Springer, New York, 2006.
doi: 10.1021/cr040695c |
|
|
(d) Sartori, G.; Maggi, R. Advances in Friedel-Crafts Acylation Reactions, CRC Press, Taylor & Francis Group, 2010.
doi: 10.1021/cr040695c |
|
| [3] |
(a) Moore, E. J.; Pretzer, W. R.; O'Connell, T. J.; Harris, J.; LaBounty, L.; Chou, L.; Grimmer, S. S. J. Am. Chem. Soc. 1992, 114, 5888.
doi: 10.1021/ja00040a078 |
|
(b) Chatani, N.; Fukuyama, T.; Kakiuchi, F.; Murai, S. J. Am. Chem. Soc. 1996, 118, 493.
doi: 10.1021/ja00040a078 |
|
|
(c) Fukuyama, T.; Chatani, N.; Kakiuchi, F.; Murai, S. J. Org. Chem. 1997, 62, 5647.
doi: 10.1021/ja00040a078 |
|
|
(d) Chatani, N.; Ie, Y.; Kakiuchi, F.; Murai, S. J. Org. Chem. 1997, 62, 2604.
doi: 10.1021/ja00040a078 |
|
|
(e) Ie, Y.; Chatani, N.; Ogo, T.; Marshall, D. R.; Fukuyama, T.; Kakiuchi, F.; Murai, S. J. Org. Chem. 2000, 65, 1475.
doi: 10.1021/ja00040a078 |
|
| [4] |
Moore E. J. Pretzer W. R. OConnell T. J. Harris J. LaBounty L. Chou L. Grimme S. S. J. Am. Chem. Soc. 1992 114 5888.
doi: 10.1021/ja00040a078 |
| [5] |
Jia X. F. Zhang S. H. Wang W. H. Luo F. Cheng J. Org. Lett. 2009 11 3120.
doi: 10.1021/ol900934g |
| [6] |
(a) Xiao, F. X.; Shuai, Q.; Zhao, F.; Basle, O.; Deng, G. J.; Li, C. J. Org. Lett. 2011, 13, 1614.
doi: 10.1021/ol200017a |
|
(b) Xu, Z. P.; Xiang, B.; Sun, P. P. RSC Adv. 2013, 3, 1679.
doi: 10.1021/ol200017a |
|
|
(c) Khemnar, A. B.; Bhanage, B. M. Eur. J. Org. Chem. 2014, 6746.
doi: 10.1021/ol200017a |
|
|
(d) Kishore, R.; Kantam, M. L.; Yadav, J.; Sudhakar, M.; Laha, S.; Venugopal, A. J. Mol. Catal. A: Chem. 2013, 379, 213.
doi: 10.1021/ol200017a |
|
|
(e) Zhang, Q.; Yang, F.; Wu, Y. J. Chem. Commun. 2013, 49, 6837.
doi: 10.1021/ol200017a |
|
|
(f) Li, M. Z.; Ge, H. B. Org. Lett. 2010, 12, 3464.
doi: 10.1021/ol200017a |
|
| [7] |
(a) Han, S.; Sharma, S.; Park, J.; Kim, M.; Shin, Y.; Mishra, N. K.; Bae, J. J.; Kwak, J. H.; Jung, Y. H.; Kim, I. S. J. Org. Chem. 2014, 79, 275.
doi: 10.1021/jo4024304 |
|
(b) Sharma, S.; Kim, M.; Park, J.; Kim, M.; Kwak, J. H.; Jung, Y. H.; Oh, J. S.; Lee, Y.; Kim, I. S. Eur. J. Org. Chem. 2013, 6656.
doi: 10.1021/jo4024304 |
|
| [8] |
Wu Y. N. Feng L. J. Lu X. Kwong F. Y. Luo H. B. Chem. Commun. 2014 50 15352.
doi: 10.1039/C4CC07440H |
| [9] |
(a) Weng, J. Q.; Yu, Z. Q.; Liu, X. H.; Zhang, G. F. Tetrahedron Lett. 2013, 54, 1205.
doi: 10.1016/j.tetlet.2012.12.059 |
|
(b) Fang, P.; Li, M. Z.; Ge, H. B. J. Am. Chem. Soc. 2010, 132, 11898.
doi: 10.1016/j.tetlet.2012.12.059 |
|
|
(c) Yin, Z. W.; Sun, P. P. J. Org. Chem. 2012, 77, 11339.
doi: 10.1016/j.tetlet.2012.12.059 |
|
|
(d) Li, C. L.; Wang, L.; Li, P. H.; Zhou, W. Chem.-Eur. J. 2011, 17, 10208.
doi: 10.1016/j.tetlet.2012.12.059 |
|
|
(e) Wu, Y. N.; Choy, P. Y.; Mao, F.; Kwong, F. Y. Chem. Commun. 2013, 49, 689.
doi: 10.1016/j.tetlet.2012.12.059 |
|
| [10] |
Yang Y. Z. Chen L. Zhang Z. G. Zhang Y. H. Org. Lett. 2011 13 1342.
doi: 10.1021/ol200025k |
| [11] |
(a) Song, H. Y.; Chen, D.; Pi, C.; Cui, X. L.; Wu, Y. J. J. Org. Chem. 2014, 79, 2955.
doi: 10.1021/jo5000219 |
|
(b) Li, H. J.; Li, P. H.; Wang, L. Org. Lett. 2013, 15, 620.
doi: 10.1021/jo5000219 |
|
|
(c) Li, H. J.; Li, P. H.; Tan, H.; Wang, L. Chem.-Eur. J. 2013, 19, 14432.
doi: 10.1021/jo5000219 |
|
|
(d) Li, Z. Y.; Li, D. D.; Wang, G. W. J. Org. Chem. 2013, 78, 10414.
doi: 10.1021/jo5000219 |
|
|
(e) Xiong, F.; Qian, C.; Lin, D. G.; Zeng, W.; Lu, X. X. Org. Lett. 2013, 15, 5444.
doi: 10.1021/jo5000219 |
|
| [12] |
Zhao J. C. Fang H. Xie C. Han J. L. Li G. G. Pan Y. Asian J. Org. Chem. 2013 2 1044.
doi: 10.1002/ajoc.201300208 |
| [13] |
(a) Ikeda, T.; Tsu, O. Science 1995, 268, 1873.
doi: 10.1126/science.268.5219.1873 |
|
(b) Kimura, K.; Suzuki, T.; Yokoyama, M. J. Phys. Chem. 1990, 94, 6090.
doi: 10.1126/science.268.5219.1873 |
|
|
(c) Campbell, D.; Dix, L. R.; Rostron, P. Dyes Pigm. 1995, 29, 77.
doi: 10.1126/science.268.5219.1873 |
|
|
(d) Huang, J. M.; Kuo, J. F.; Chen, C. Y. J. Appl. Polym. Sci. 1995, 55, 1217.
doi: 10.1126/science.268.5219.1873 |
|
|
(e) Lee, H. K.; Kanazawa, A.; Shiono, T.; Ikeda, T.; Fujisawa, T.; Aizawa, M.; Lee, B. Chem. Mater. 1998, 10, 1402.
doi: 10.1126/science.268.5219.1873 |
|
|
(f) Li, H. J.; Li, P. H.; Zhao, Q.; Wang, L. Chem. Commun. 2013, 49, 9170.
doi: 10.1126/science.268.5219.1873 |
|
| [14] |
For selected examples, see: (a) Hou, Z.; Fujiware, Y.; Taniguchi, H. J. Org. Chem. 1988, 53, 3118.
doi: 10.6023/cjoc201205026 |
|
(b) Sakai, N.; Fuji, K.; Nabeshima, S.; Ikeda, R.; Konakahara, T. Chem. Commun. 2010, 46, 3173.
doi: 10.6023/cjoc201205026 |
|
|
(c) Wada, S.; Urano, M.; Suzuki, H. J. Org. Chem. 2002, 67, 8254.
doi: 10.6023/cjoc201205026 |
|
|
(d) Wang, Y.; Cheng, G. L.; Cui, X. L. Chin. J. Org. Chem. 2012, 32, 2018 (in Chinese).
doi: 10.6023/cjoc201205026 |
|
|
(王勇, 程国林, 崔秀灵, 有机化学, 2012, 32, 2018.)
doi: 10.6023/cjoc201205026 |
|
| [15] |
(a) Sun, M.; Hou, L. K.; Chen, X. X.; Yang, X. J.; Sun, W.; Zang, Y. S. Adv. Synth. Catal. 2014, 356, 3789.
doi: 10.1002/adsc.201400594 |
|
(b) Li, H. J.; Li, P. H.; Zhao, Q.; Wang, L. Chem. Commun. 2013, 49, 9170.
doi: 10.1002/adsc.201400594 |
|
|
(c) Yi, M. L.; Cui, X. L.; Zhu, C. W.; Pi, C.; Zhu, W. M.; Wu, Y. J. Asian J. Org. Chem. 2015, 4, 38.
doi: 10.1002/adsc.201400594 |
|
|
(d) Hou, L. K.; Chen, X. X.; Li, S.; Cai, S. X.; Zhao, Y. X.; Sun, M.; Yang, X. J. Org. Biomol. Chem., 2015, 13, 4160.
doi: 10.1002/adsc.201400594 |
|
| [16] |
(a) Yang, J.; Fu, T.; Long, Y.; Zhou, X. G. Chin. J. Org. Chem. 2017, 37, 1111 (in Chinese).
doi: 10.6023/cjoc201702045 |
|
(杨军, 付婷, 龙洋, 周向葛, 有机化学, 2017, 37, 1111.)
doi: 10.6023/cjoc201702045 |
|
|
(b) Zhou, Z.; Duan, J. F.; Mu, X. J.; Xiao, S. Y. Chin. J. Org. Chem. 2018, 38, 585 (in Chinese).
doi: 10.6023/cjoc201702045 |
|
|
(周曌, 段建凤, 穆小静, 肖尚友, 有机化学, 2018, 38, 585.)
doi: 10.6023/cjoc201702045 |
|
|
(c) Qin, H. F.; Li, X. R. Chin. J. Org. Chem. 1992, 12, 309 (in Chinese).
doi: 10.6023/cjoc201702045 |
|
|
(秦合法, 李萱荣, 有机化学, 1992, 12, 309.)
doi: 10.6023/cjoc201702045 |
|
| [17] |
(a) Szabó, F.; Daru, J.; Simkó, D.; Nagy, T. Z.; Stirling, A.; Novák, Z. Adv. Synth. Catal. 2013, 355, 685.
doi: 10.1002/adsc.201200948 |
|
(b) Szabó, F.; Simkó, D.; Novák, Z. RSC Adv. 2014, 4, 3883.
doi: 10.1002/adsc.201200948 |
|
|
(c) Xiao, F. H.; Chen, S. Q.; Huang, H. W.; Deng, G. J. Eur. J. Org. Chem. 2015, 7919.
doi: 10.1002/adsc.201200948 |
|
| [18] |
Zhang D. Cui X. L. Yang F. F. Q. Zhang Q. Q. Zhu Y. Wu Y. J. Org. Chem. Front. 2015 2 951.
doi: 10.1039/C5QO00120J |
| [19] |
(a) Rosewall, C. F.; Sibbald, P. A.; Liskin, D. V.; Michael, F. E. J. Am. Chem. Soc. 2009, 131, 9488.
doi: 10.1021/ja9031659 |
|
(b) Xu, L. M.; Li, B. J.; Yang, Z.; Shi, Z. J. Chem. Soc. Rev. 2010, 39, 712.
doi: 10.1021/ja9031659 |
|
|
(c) Sibbald, P. A.; Rosewall, C. F.; Swartz, R. D.; Michael, F. E. J. Am. Chem. Soc. 2009, 131, 15945.
doi: 10.1021/ja9031659 |
|
|
(d) Powers, D. C.; Ritter, T. Nat. Chem. 2009, 1, 302.
doi: 10.1021/ja9031659 |
|
|
(e) Powers, D. C.; Geibel, M. A. L.; Klein, J. E. M. N.; Ritter, T. J. Am. Chem. Soc. 2009, 131, 17050.
doi: 10.1021/ja9031659 |
|
|
(f) Deprez, N. R.; Sanford, M. S. J. Am. Chem. Soc. 2009, 131, 11234.
doi: 10.1021/ja9031659 |
|
|
(g) Racowski, J. M.; Dick, A. R.; Sanford, M. S. J. Am. Chem. Soc. 2009, 131, 10974.
doi: 10.1021/ja9031659 |
|
| [20] | Christin G. Beate P. Elisabeth I. Karola R. B. Synthesis 2008 1889. |
| [1] | 黄净, 杨毅华, 张占辉, 刘守信. 酰胺键的绿色高效构建方法与技术进展[J]. 有机化学, 2024, 44(2): 409-420. |
| [2] | 孟宪强, 杨艺, 梁万洁, 王靖涛, 张荣葵, 刘会. 钯催化联烯胺区域选择性芳基酚氧化反应[J]. 有机化学, 2024, 44(1): 224-231. |
| [3] | 蒋宜欣, 唐伯孝, 毛海波, 陈雪霞, 俞洋杰, 全翠英, 徐昭阳, 石金慧, 刘益林. 水-聚乙二醇(PEG-200)中烯烃与碘代芳烃绿色可循环无负载偶联反应的研究[J]. 有机化学, 2023, 43(9): 3210-3215. |
| [4] | 王兢睿, 冯永奎, 王能中, 黄年玉, 姚辉. 钯催化立体选择性合成硝基烷类β-碳糖苷[J]. 有机化学, 2023, 43(9): 3216-3225. |
| [5] | 岁丹丹, 岑南楠, 龚若蕖, 陈阳, 陈文博. 无支持电解质条件下连续流电化学合成三氟甲基化氧化吲哚[J]. 有机化学, 2023, 43(9): 3239-3245. |
| [6] | 王玉超, 刘晋彪, 何智涛. 钯催化共轭二烯的不对称氢官能团化[J]. 有机化学, 2023, 43(8): 2614-2627. |
| [7] | 卢凯, 屈浩琦, 陈樨, 秋慧, 郑晶, 马猛涛. 无催化剂、无溶剂条件下炔烃和烯烃与儿茶酚硼烷的硼氢化反应[J]. 有机化学, 2023, 43(6): 2197-2205. |
| [8] | 窦谦, 汪太民, 房丽晶, 翟宏斌, 程斌. 光诱导铁催化在有机合成中的应用研究进展[J]. 有机化学, 2023, 43(4): 1386-1415. |
| [9] | 莫百川, 陈春霞, 彭进松. 木质素及其衍生物负载金属催化剂在有机合成中的应用研究进展[J]. 有机化学, 2023, 43(4): 1215-1240. |
| [10] | 张妍妍, 张珠珠, 朱圣卿, 储玲玲. 镍催化不对称酰基化反应研究进展[J]. 有机化学, 2023, 43(3): 1023-1035. |
| [11] | 向勋, 何照林, 董秀琴. 钯和手性磷酸协同催化高效构建手性分子的研究进展[J]. 有机化学, 2023, 43(3): 791-808. |
| [12] | 孙美娇, 谭晶, 谭玉, 彭进松, 陈春霞. 钯催化3-(2-氨基嘧啶-4-基)吲哚2位C—H键芳基化反应的研究[J]. 有机化学, 2023, 43(11): 3945-3959. |
| [13] | 李奇阳, 张海燕, 刘文博. 无过渡金属参与的碳硅键构筑方法研究进展[J]. 有机化学, 2023, 43(10): 3470-3490. |
| [14] | 熊威, 石斌, 姜烜, 陆良秋, 肖文精. 配体调控钯催化乙烯基环状碳酰胺和异氰酸酯的差异性转化[J]. 有机化学, 2023, 43(1): 265-273. |
| [15] | 魏琬絜, 詹磊, 高雷, 黄国保, 马献力. 电化学合成C-磺酰基化合物的研究进展[J]. 有机化学, 2023, 43(1): 17-35. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||