有机化学 ›› 2021, Vol. 41 ›› Issue (3): 969-982.DOI: 10.6023/cjoc202007020 上一篇 下一篇
所属专题: 热点论文虚拟合集
综述与进展
收稿日期:2020-07-07
修回日期:2020-09-01
发布日期:2020-10-12
通讯作者:
伍婉卿
基金资助:
Yujie Xiaa, Dandan Hea, Wanqing Wua,b,*( )
)
Received:2020-07-07
Revised:2020-09-01
Published:2020-10-12
Contact:
Wanqing Wu
About author:Supported by:文章分享

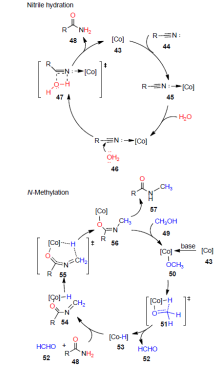
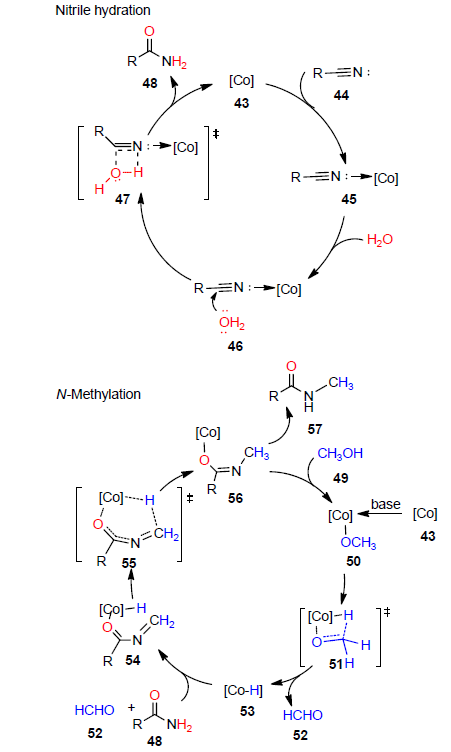
腈可用于构建新的碳-碳、碳-杂原子键, 所得产物丰富多样. 酰胺基团广泛存在于医药、农药和天然产物中, 此外, 酰胺还是有机合成反应中重要的中间体. 在目前报道的酰胺类化合物的合成方法中, 腈的水合反应已成为学术界和工业界最广泛使用的获得初级酰胺类化合物的方法之一. 早期腈的水合反应中通常涉及强酸、强碱的使用, 但在该类反应体系下, 往往存在产率低及反应选择性差等问题, 且所得酰胺容易过度水解成羧酸. 为了克服这一局限实现腈的高效水合, 且满足绿色化学的要求, 近年来, 不同的催化体系相继被开发, 如过渡金属配合物催化剂、金属阳离子催化剂、金属纳米粒子催化剂、离子液体催化剂及其它类型催化剂. 对这些催化体系下腈的水合反应研究进行阐述和总结, 并对该领域的发展前景进行了展望.
夏玉杰, 何丹丹, 伍婉卿. 不同催化体系下腈的水合反应研究进展[J]. 有机化学, 2021, 41(3): 969-982.
Yujie Xia, Dandan He, Wanqing Wu. Recent Advances for Hydration Reaction of Nitriles in Different Catalytic Systems[J]. Chinese Journal of Organic Chemistry, 2021, 41(3): 969-982.
| [1] |
Song, C.-Y.; Qu, S.-L; Tao, Y.; Dang, Y.-F; Wang, Z.-X. ACS Catal. 2014, 4, 2854. d4bf9322-6a9d-4703-a4a6-93fe27165b27
doi: 10.1021/cs5008156 |
| [2] |
Pattabiraman, V. R.; Bode, J. W. Nature 2011, 480, 471.
pmid: 22193101 |
| [3] |
Gunanathan, C.; Ben-David, Y.; Milstein, D. Science 2007, 317, 790.
pmid: 17690291 |
| [4] |
Mahjour, B.; Shen, Y.; Liu, W.; Cernak, T. Nature 2020, 580, 71.
pmid: 32238943 |
| [5] |
García-Álvarez, R.; Francos, J.; Tomás-Mendivil, E.; Crochet, P.; Cadierno, V. J. Org. Chem. 2014, 79, 93.
|
| [6] |
Wang, Y.; Chen, C.; Zhang, S.; Lou, Z.-B.; Su, X.; Wen, L.-R.; Li, M. Org. Lett. 2013, 15, 4794.
pmid: 24015723 |
| [7] |
Ma, B.; Wang, Y.; Peng, J.; Zhu, Q. J. Org. Chem. 2011, 76, 6362.
doi: 10.1021/jo2007362 pmid: 21675775 |
| [8] |
Wu, Z.; Ren, R.; Zhu, C. Angew. Chem., Int. Ed. 2016, 55, 10821.
|
| [9] |
Tílvez, E.; Menéndez, M. I.; López, R. Organometallics 2012, 31, 1618.
|
| [10] |
Lee, J.; Kim, M.; Chang, S.; Lee, H. Y. Org. Lett. 2009, 11, 5598.
pmid: 19911824 |
| [11] |
Guo, B.; de Vries, J. G.; Otten, E. Chem. Sci. 2019, 10, 10647.
doi: 10.1039/c9sc04624k pmid: 32110350 |
| [12] |
Ahmed, T. J.; Knapp, S. M. M.; Tyler, D. R. Coord. Chem. Rev. 2011, 255, 949.
|
| [13] |
Kim, J. H. Angew. Chem., Int. Ed. 1990, 29, 523.
doi: 10.1002/(ISSN)1521-3773 |
| [14] |
Diomand, S. E.; Grant, B.; Tom, G. M. Tetrahedron Lett. 1974, 15, 4025.
doi: 10.1016/S0040-4039(01)92074-X |
| [15] |
Murahashi, S. I.; Naota, T. Bull. Agric. Chem. Soc. Jpn. 1996, 69, 1805.
|
| [16] |
García-Álvarez, R.; Crochet, P.; Cadierno, V. Green Chem. 2013, 15, 46.
|
| [17] |
Fung, W. K.; Huang, X.; Man, M. L.; Ng, S. M.; Hung, M. Y.; Lin, Z.; Lau, C. P. J. Am.Chem. Soc. 2003, 125, 11539.
doi: 10.1021/ja034050q |
| [18] |
Cadierno, V.; Diez, J.; Francos, J.; Gimeno, J. Chem.-Eur. J. 2010, 16, 9808.
pmid: 20586085 |
| [19] |
García-Álvarez, R.; Díez, J.; Crochet, P.; Cadierno, V. Organometallics 2011, 30, 5442.
|
| [20] |
Knapp, S. M. M.; Sherbow, T. J.; Yelle, R. B.; Zakharov, L. N.; Juliette, J. J.; Tyler, D. R. Organometallics 2013, 32, 824.
|
| [21] |
Tomás-Mendivil, E.; García-Álvarez, R.; Vidal, C.; Crochet, P.; Cadierno, V. ACS Catal. 2014, 4, 1901.
|
| [22] |
Tomás-Mendivil, E.; Menéndez-Rodríguez, L.; Francos, J.; Crochet, P.; Cadierno, V. RSC Adv. 2014, 4, 63466.
|
| [23] |
Geldbach, T. J.; Drago, D.; Pregosin, P. S. Chem. Commun. 2000,1629.
|
| [24] |
Geldbach, T. J.; Breher, F.; Gramlich, V.; Kumar, P. G. A.; Pregosin, P. S. Inorg. Chem. 2004, 43, 1920.
pmid: 15018511 |
| [25] |
Leung, C. W.; Zheng, W. X.; Wang, D. X.; Ng, S. M.; Yeung, C. H.; Zhou, Z. Y.; Lin, Z. Y.; Lau, C. P. Organometallics 2007, 26, 1924.
|
| [26] |
Tomás-Mendivil, E.; Suárez, F. J.; Díez, J.; Cadierno, V. Chem. Commun. 2014, 50, 9661.
|
| [27] |
Ghaffar, T.; Parkins, A. W. Tetrahedron Lett. 1995, 36, 8657.
doi: 10.1016/0040-4039(95)01785-G |
| [28] |
Xing, X.-Y.; Xu, C.; Chen, B.; Li, C.-C.; Virgil, S. C.; Grubbs, R. H. J. Am. Chem. Soc. 2018, 140, 17782.
doi: 10.1021/jacs.8b11667 pmid: 30482014 |
| [29] |
Paul, B.; Maji, M.; Kundu, S. ACS Catal. 2019, 9, 10469.
|
| [30] |
Breno, K. L.; Pluth, M. D.; Tyler, D. R. Organometallics 2003, 22, 1203.
|
| [31] |
Takaya, H.; Yoshida, K.; Isozaki, K.; Terai, H.; Murahashi, S. Angew. Chem., Int. Ed. 2003, 42, 3302.
|
| [32] |
Goto, A.; Endo, K.; Saito, S. Angew. Chem., Int. Ed. 2008, 47, 3607.
|
| [33] |
Anderson, N. H.; Boncella, J. M.; Tondreau, A. M. Organometallics 2018, 37, 4675.
|
| [34] |
Kim, E. S.; Kim, H. S.; Kim, J. N. Tetrahedron Lett. 2009, 50, 2973.
|
| [35] |
Kim, E. S.; Lee, H. S.; Kim, S. H.; Kim, J. N. Tetrahedron Lett. 2010, 51, 1589.
|
| [36] |
Ma, X.-Y.; He, Y.; Hu, Y.-L.; Lu, M. Tetrahedron Lett. 2012, 53, 449.
|
| [37] |
Ma, X.-Y.; He, Y.; Wang, P.-C.; Lu, M. Appl. Organomet. Chem. 2012, 26, 377.
|
| [38] |
Ma, X.-Y.; He, Y.; Lu, M. Synth. Commun. 2013, 44, 474.
|
| [39] |
Sanz Sharley, D.D.; Williams,, J. M. J. Tetrahedron Lett. 2017, 58, 4090.
|
| [40] |
Kanda, T.; Naraoka, A.; Naka, H. J. Am. Chem. Soc. 2019, 141, 825.
pmid: 30590921 |
| [41] |
Oberhauser, W.; Bartoli, M.; Petrucci, G.; Bandelli, D. J. Mol. Catal. A: Chem. 2015, 410, 26.
|
| [42] |
Mitsudome, T.; Mikami, Y.; Mori, H.; Arita, S.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Chem. Commun. 2009,3258.
|
| [43] |
Woo, H.; Lee, K.; Park, S.; Park, K. H. Molecules 2014, 19, 699.
pmid: 24402201 |
| [44] |
Kim, A. Y.; Bae, H. S.; Park, S.; Park, S.; Park, K. H. Catal. Lett. 2011, 141, 685.
|
| [45] |
Shimizu, K.-I.; Imaiida, N.; Sawabe, K.; Satsuma, A. Appl. Catal., A. 2012, 421~ 422 , 114.
|
| [46] |
Gangarajula, Y.; Gopal, B. Appl. Catal., A 2014, 475, 211.
|
| [47] |
Baig, R. B.; Varma, R. S. Chem. Commun. 2012, 48, 6220.
|
| [48] |
Yan, N.; Xiao, C.-X.; Kou, Y. Coord. Chem. Rev. 2010, 254, 1179.
|
| [49] |
Gong, J. L.; Mullins, C. B. Acc. Chem. Res. 2009, 42, 1063.
pmid: 19588952 |
| [50] |
Gladys, M. J.; El Zein, A. A.; Mikkelsen, A.; Andersen, J. N.; Held, G. Surf. Sci. 2008, 602, 3540.
|
| [51] |
Pan, M.; Hoang, S.; Mullins, C. B. Catal. Today 2011, 160, 198.
doi: 10.1016/j.cattod.2010.05.008 |
| [52] |
Shimizu, K.-I.; Kubo, T.; Satsuma, A.; Kamachi, T.; Yoshizawa, K. ACS Catal. 2012, 2, 2467. aff1e2c8-5286-43fd-b6b9-837d53bc53bf
doi: 10.1021/cs3006154 |
| [53] |
Sherbow, T. J.; Downs, E. L.; Sayler, R. I.; Razink, J. J.; Juliette, J. J.; Tyler, D. R. ACS Catal. 2014, 4, 3096.
doi: 10.1021/cs500830s |
| [54] |
Mulfinger, L.; Solomon, S. D.; Bahadory, M.; Jeyarajasingam, A. V.; Rutkowsky, S. A.; Boritz, C. J. Chem. Educ. 2007, 84, 322.
|
| [55] |
Liu, Y.-M.; He, L.; Wang, M.-M.; Cao, Y.; He, H.-Y.; Fan, K.-N. ChemSusChem 2012, 5, 1392.
doi: 10.1002/cssc.201200203 pmid: 22674755 |
| [56] |
Kumar, S.; Sharma, S.; Das, P. Adv. Synth. Catal. 2016, 358, 2889.
|
| [57] |
Mehta, A.; Basu, S. J. Photochem. Photobiol., A 2017, 343, 1.
|
| [58] |
Wang, H.; Wang, Y.-Q.; Xu, H.; Zhou, H.; Wang, L.; Meng, X.-J.; Xiao, F.-S. Ind. Eng. Chem. Res. 2019, 58, 17319.
|
| [59] |
Goossens, K.; Lava, K.; Bielawski, C. W.; Binnemans, K. Chem. Rev. 2016, 116, 4643.
doi: 10.1021/cr400334b pmid: 27088310 |
| [60] |
Rantwijk, F. V.; Sheldon, R. A. Chem. Rev. 2007, 107, 2757.
pmid: 17564484 |
| [61] |
Han, X. X.; Armstrong, D. W. Acc. Chem. Res. 2007, 40, 1079.
pmid: 17910515 |
| [62] |
Veisi, H.; Manesh, A. A.; Khankhani, N.; Ghorbani-Vaghei, R. RSC Adv. 2014, 4, 25057.
|
| [63] |
Earle, M. J.; Esperanca, J. M.; Gilea, M. A.; Lopes, J. N.; Rebelo, L. P.; Magee, J. W.; Seddon, K. R.; Widegren, J. A. Nature 2006, 439, 831.
pmid: 16482154 |
| [64] |
Dong, K.; Liu, X.-M.; Dong, H.-F.; Zhang, X.-P.; Zhang, S.-J. Chem. Rev. 2017, 117, 6636.
doi: 10.1021/acs.chemrev.6b00776 pmid: 28488441 |
| [65] |
Kalkhambkar, R. G.; Waters, S. N.; Laali, K. K. Tetrahedron Lett. 2011, 52, 867.
|
| [66] |
Kumar, S.; Dixit, S. K.; Awasthi, S. K. Tetrahedron Lett. 2014, 55, 3802.
|
| [67] |
Veisi, H.; Maleki, B.; Hamelian, M.; Ashrafi, S. S. RSC Adv. 2015, 5, 6365.
|
| [68] |
Dutta, A.; Damarla, K.; Kumar, A.; Saikia, P. J.; Sarma, D. Tetrahedron Lett. 2020, 61, 151587.
|
| [69] |
Tu, T.; Wang, Z.-X.; Liu, Z. L.; Feng, X.-K.; Wang, Q.-Y. Green Chem. 2012, 14, 921.
|
| [70] |
Chen, H.-N.; Dai, W.-J.; Chen, Y.; Xu, Q.; Chen, J.-H.; Yu, L.; Zhao, Y.-J.; Ye, M.-D.; Pan, Y.-J. Green Chem. 2014, 16, 2136.
|
| [71] |
Midya, G. C.; Kapat, A.; Maiti, S.; Dash, J. J. Org. Chem. 2015, 80, 4148.
doi: 10.1021/jo502752u pmid: 25786059 |
| [72] |
Chitale, S.; Derasp, J. S.; Hussain, B.; Tanveer, K.; Beauchemin, A. M. Chem. Commun. 2016, 52, 13147.
|
| [1] | 高宝昌, 石雨, 田媛, 张治国, 张婧如, 孙宇峰, 毛国梁, 戴凌燕. 4-甲基-2-氧代-6-芳氨基-二氢-吡喃-3-腈衍生物的合成[J]. 有机化学, 2024, 44(2): 644-649. |
| [2] | 张勇, 田志高, 黄琳, 侯秋飞, 范红红, 汪万强. α-氰醇甲磺酸酯在合成α-氨基腈类化合物中的应用[J]. 有机化学, 2024, 44(2): 561-572. |
| [3] | 陶苏艳, 项紫欣, 白俊杰, 万潇, 万小兵. 亚硝酸叔丁酯参与的酰胺水解反应[J]. 有机化学, 2024, 44(2): 550-560. |
| [4] | 江港钟, 林嘉欣, 鲍晓光, 万小兵. 亚硝酸异戊酯活化伯磺酰胺制备磺酰溴与磺酰氯[J]. 有机化学, 2024, 44(2): 533-549. |
| [5] | 黄净, 杨毅华, 张占辉, 刘守信. 酰胺键的绿色高效构建方法与技术进展[J]. 有机化学, 2024, 44(2): 409-420. |
| [6] | 李洋, 董亚楠, 李跃辉. 经由N-硼基酰胺中间体的酰胺高效转化合成腈类化合物[J]. 有机化学, 2024, 44(2): 638-643. |
| [7] | 李鹏辉, 谢青洋, 万福贤, 张元红, 姜林. 含环丙基的新型取代嘧啶-5-甲酰胺的合成及杀菌活性研究[J]. 有机化学, 2024, 44(2): 650-656. |
| [8] | 黄志友, 杨平, 何波, 欧文霞, 袁思雨. 吗啉磺酰胺化合物的设计、合成及其抑制大豆萌芽活性的研究[J]. 有机化学, 2024, 44(1): 309-315. |
| [9] | 徐利军, 李宗军, 韩福社, 高翔. N,N-二甲基甲酰胺促进的富勒烯稠合噁唑啉衍生物的合成[J]. 有机化学, 2024, 44(1): 242-250. |
| [10] | 王博珍, 张婕, 粘春惠, 金茗茗, 孔苗苗, 李物兰, 何文斐, 吴建章. 含有3,4-二氯苯基的酰胺类化合物的合成及抗肿瘤活性研究[J]. 有机化学, 2024, 44(1): 232-241. |
| [11] | 唐菁, 罗文坤, 周俊. 氮杂螺[4.5]三烯酮衍生物的合成研究进展[J]. 有机化学, 2023, 43(9): 3006-3034. |
| [12] | 贝文峰, 潘健, 冉冬梅, 刘伊琳, 杨震, 冯若昆. 基于钴催化吲哚酰胺与二炔和单炔的[4+2]环化反应合成γ-咔啉酮[J]. 有机化学, 2023, 43(9): 3226-3238. |
| [13] | 马虎, 黄丹凤, 王克虎, 唐朵朵, 冯杨, 任园园, 王君娇, 胡雨来. 3-(三氟甲基)吡唑类化合物的合成[J]. 有机化学, 2023, 43(9): 3257-3267. |
| [14] | 张俊杰, 徐学涛. (S)-(–)-Xylopinine和(S)-(+)-Laudanosine的不对称合成[J]. 有机化学, 2023, 43(9): 3297-3303. |
| [15] | 席敏, 段超, 迟捷, 付甜, 苏小龙, 王宏社. 腐殖酸作用下Strecker反应快速高效合成α-氨基腈[J]. 有机化学, 2023, 43(9): 3312-3318. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||