有机化学 ›› 2021, Vol. 41 ›› Issue (4): 1498-1526.DOI: 10.6023/cjoc202010002 上一篇 下一篇
综述与进展
侯秋飞a, 张锐a, 梁洁媚a, 姚维忠a, 程华a,*( ), 陈宬b,*(
), 陈宬b,*( )
)
收稿日期:2020-10-02
修回日期:2020-10-27
发布日期:2020-11-04
通讯作者:
程华, 陈宬
基金资助:
Qiufei Houa, Rui Zhanga, Jiemei Lianga, Weizhong Yaoa, Hua Chenga,*( ), Cheng Chenb,*(
), Cheng Chenb,*( )
)
Received:2020-10-02
Revised:2020-10-27
Published:2020-11-04
Contact:
Hua Cheng, Cheng Chen
About author:Supported by:文章分享

2-氨基苯并噻唑类化合物是一类极其重要的氮杂环化合物, 在医药、农药等领域具有广阔的应用前景. 鉴于这类化合物多样的生物活性, 科研工作者们广泛关注并开发出很多新颖的合成方法. 系统总结了2-氨基苯并噻唑类化合物的合成方法, 按照合成原料的不同对这些方法进行了归类和分析, 并对其未来发展进行了展望.
侯秋飞, 张锐, 梁洁媚, 姚维忠, 程华, 陈宬. 2-氨基苯并噻唑类化合物的合成研究进展[J]. 有机化学, 2021, 41(4): 1498-1526.
Qiufei Hou, Rui Zhang, Jiemei Liang, Weizhong Yao, Hua Cheng, Cheng Chen. Progress in the Synthesis of 2-Aminobenzothiazoles[J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1498-1526.
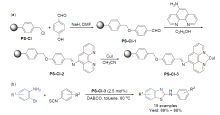
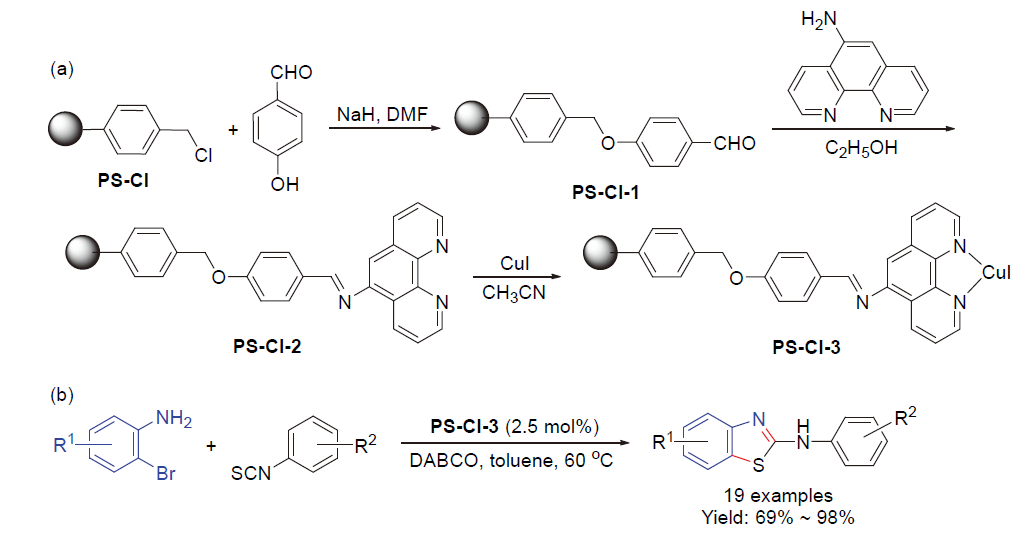

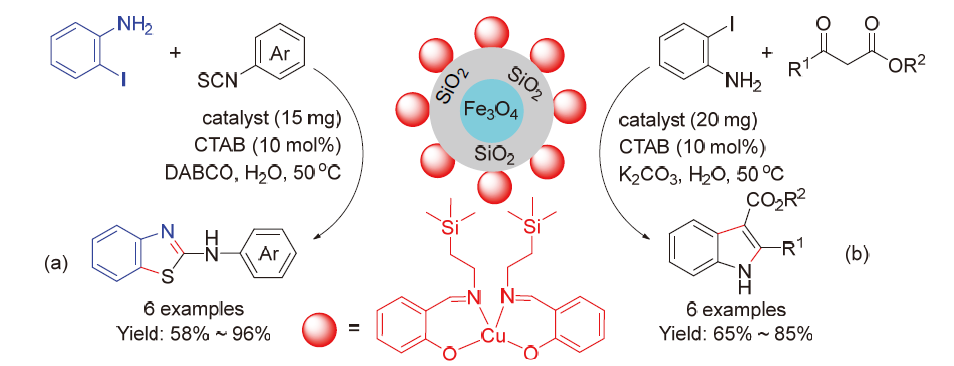
| [1] |
Gill, R.K.; Rawal, R.K.; Bariwal, J. Arch. Pharm. Chem. Life Sci. 2015, 348,155.
|
| [2] |
Facchinetti, V.; Reis, R.R.; Gomes, C.R. B.; Vasconcelos, T.R. A. Mini-Rev. Org. Chem. 2012, 9,44.
|
| [3] |
Pathak, N.; Rathi, E.; Kumar, N.; Kini, S.G.; Rao, C.M. Mini-Rev. Med. Chem. 2020, 20,12.
pmid: 31288719 |
| [4] |
Irfan, A.; Batool, F.; Naqvi, S.A. Z.; Islam, A.; Osman, S.M.; Nocentini, A.; Alissa, S.A.; Supuran, C.T. J. Enzyme Inhib. Med. Chem. 2020, 35,265.
|
| [5] |
Sharma, P.C.; Bansal, K.K.; Deep, A.; Pathak, M. Curr. Top. Med. Chem. 2017, 17,208.
|
| [6] |
Gagoria, J.; Verma, P.K.; Khatkar, A. Cent. Nerv. Syst. Agents Med. Chem. 2015, 15,11.
|
| [7] |
Jiang, K.; Cao, L.; Hao, Z.-F.; Chen, M.Y.; Cheng, J.-L.; Li, X.; Xiao, P.; Chen, L.; Wang, Z.-Y. Chin. J. Org. Chem. 2017, 37,2221. (in Chinese)
|
|
( 蒋凯, 曹梁, 郝志峰, 陈美燕, 程洁銮, 李晓, 肖萍, 陈亮, 汪朝阳, 有机化学, 2017, 37,2221.)
|
|
| [8] |
Ballari, M.S.; Cano, N.H.; Lopez, A.G.; Wunderlin, D.A.; Feresin, G.; Santiago, A.N. J. Agric. Food Chem. 2017, 65,10325.
|
| [9] |
Si, W.-J.; Chen, M.; Wang, X.-L.; Wang, M.-Q.; Jiao, J.; Fu, X.-C.; Yang, C.-L. ARKIVOC 2018, 7,86.
|
| [10] |
Tariq, S.; Kamboj, P.; Amir, M. Arch. Pharm. Chem. Life Sci. 2018, 352,e1800170.
|
| [11] |
Kamal, A.; Syed, M.A. H.; Mohammed, S.M. Expert Opin. Ther. Pat. 2015, 25,355.
|
| [12] |
Keri, R.S.; Patil, M.R.; Patil, S.A.; Budagumpi, S. Eur. J. Med. Chem. 2015, 89,207.
pmid: 25462241 |
| [13] |
Hu, Y.-X.; Xia, X.; He, W.-Z.; Tang, Z.-J.; Lv, Y.-L.; Li, X.; Zhang, D.-Y. Org. Electron. 2019, 66,126.
|
| [14] |
Zhang, M.; Liang, G. Sci. China: Chem. 2018, 61,1088.
|
| [15] |
Maruszewska, A.; Podsiadly, R. Color. Technol. 2014, 130,243.
|
| [16] |
Ghosh, P.; Katare, S.; Patkar, P.; Caruthers, J.M.; Venkatasubramanian, V.; Walker, K.A. Rubber Chem. Technol. 2003, 76,592.
|
| [17] |
Orlita, A. Int. Biodeterior. Biodegrad. 2004, 53,157.
|
| [18] |
Liao, C.; Kim, U.-J.; Kannan, K. Environ. Sci. Technol. 2018, 52,5007.
|
| [19] |
Li, Y.; Wang, Y.-L. Chin. J. Org. Chem. 2006, 26,878. (in Chinese)
|
|
( 李焱, 王玉炉, 有机化学, 2006, 26,878.)
|
|
| [20] |
Noel, S.; Cadet, S.; Gras, E.; Hureau, C. Chem. Soc. Rev. 2013, 42,7747.
|
| [21] |
Zhu, N.; Zhang, Z.-W.; Gao, M.; Han, L.-M.; Suo, Q.-L.; Hong, H.-L. Chin. J. Org. Chem. 2013, 33,1423. (in Chinese)
|
|
( 竺宁, 张志伟, 高敏, 韩利民, 索全伶, 洪海龙, 有机化学, 2013, 33,1423.)
|
|
| [22] |
Prajapati, N.P.; Vekariya, R.H.; Borad, M.A.; Patel, H.D. RSC Adv. 2014, 4,60176.
|
| [23] |
Dai, X.-Q.; Zhu, Y.-B.; Wang, Z.-Y.; Weng, J.-Q. Chin. J. Org. Chem. 2017, 37,1924. (in Chinese)
|
|
( 戴小强, 朱亚波, 汪洲洋, 翁建全, 有机化学, 2017, 37,1924.)
|
|
| [24] |
Liu, X.; Dong, Z.-B. Eur. J. Org. Chem. 2020, 2020,408.
|
| [25] |
Gan, F.; Luo, P.; Lin, J.; Ding, Q. Synthesis 2020,DOI: 10.1055/s-0040-1707208.
|
| [26] |
Sweeney, J.B.; Rattray, M.; Pugh, V.; Powell, L.A. ACS Med. Chem. Lett. 2018, 9,552.
|
| [27] |
Navarro, S.; Bermejo, S.; Vela, N.; Hernández, J. J. Agric. Food Chem. 2009, 57,6375.
|
| [28] |
Verfaille, C.J.; Borgers, M.; Steensel, M.A. M. J. Dtsch. Dermatol. Ges. 2008, 6,355.
|
| [29] |
Baert, B.; Spiegeleer, B.D. Skin Pharmacol. Physiol. 2011, 24,151.
|
| [30] |
Xie, Y.; Deng, S.; Chen, Z.; Yan, S.; Landry, D.W. Bioorg. Med. Chem. Lett. 2006, 16,4657.
|
| [31] |
Hroch, L.; Guest, P.; Benek, O.; Soukup, O.; Janockova, J.; Dolezal, R.; Kuca, K.; Aitken, L.; Smith, T.K.; Gunn-Moore, F.; Zala, D.; Ramsay, R.R.; Musilek, K. Bioorg. Med. Chem. 2017, 25,1143.
|
| [32] |
Koppireddi, S.; Komsani, J.R.; Avula, S.; Pombala, S.; Vasamsetti, S.; Kotamraju, S.; Yadla, R. Eur. J. Med. Chem. 2013, 66,305.
|
| [33] |
Haroun, M.; Tratrat, C.; Kositsi, K.; Tsolaki, E.; Petrou, A.; Aldhubiab, B.; Attimarad, M.; Harsha, S.; Geronikaki, A.; Venugopala, K.N.; Elsewedy, H.S.; Sokovic, M.; Glamoclija, J.; Ciric, A. Curr. Top. Med. Chem. 2018, 18,75.
|
| [34] |
Thakkar, S.S.; Thakor, P.; Ray, A.; Doshi, H.; Thakkar, V.R. Bioorg. Med. Chem. 2017, 25,5396.
|
| [35] |
Xiao, H.; Li, P.; Hu, D.; Song, B.A. Bioorg. Med. Chem. Lett. 2014, 24,3452.
|
| [36] |
Siddiqui, N.; Alam, M.S.; Sahu, M.; Naim, M.J.; Yar, M.S.; Alam, O. Bioorg. Chem. 2017, 71,230.
|
| [37] |
Telvekar, V.N.; Bairwa, V.; Satardekar, K.; Bellubi, A. Bioorg. Med. Chem. Lett. 2012, 22,649.
pmid: 22079026 |
| [38] |
Catalano, A.; Carocci, A.; Defrenza, I.; Muraglia, M.; Carrieri, A.; Bambeke, F.V.; Rosato, A.; Corbo, F.; Franchini, C. Eur. J. Med. Chem. 2013, 64,357.
|
| [39] |
Navarrete-Vazquez, G.; Ramírez-Martínez, M.; Estrada-Soto, S.; Nava-Zuazo, C.; Paoli, C.; Camici, G.; Escalante-García, J.; Medina-Franco, J.L.; Lόpez-Vallejo, F.; Ortiz-Andrade, R. Eur. J. Med. Chem. 2012, 53,346.
pmid: 22583779 |
| [40] |
Venugopala, K.N.; Krishnappa, M.; Nayak, S.K.; Subrahmanya, B.K.; Vaderapura, J.P.; Chalannavar, R.K.; Gleiser, R.M.; Odhav, B. Eur. J. Med. Chem. 2013, 65,295.
|
| [41] |
Tariq, S.; Kamboj, P.; Alam, O.; Amir, M. Bioorg. Chem. 2018, 81,630.
|
| [42] |
Pitta, E.; Geronikaki, A.; Surmava, S.; Eleftheriou, P.; Mehta, V.P.; Van der Eycken, E.V. J. Enzyme Inhib. Med. Chem. 2013, 28,113.
|
| [43] |
Noolvi, M.N.; Patel, H.M.; Kaur, M. Eur. J. Med. Chem. 2012, 54,447.
|
| [44] |
Jordan, A.D.; Luo, C.; Reitz, A.B. J. Org. Chem. 2003, 68,8693.
pmid: 14575503 |
| [45] |
Dadmal, T.L.; Katre, S.D.; Mandewale, M.C.; Kumbhare, R.M. New J. Chem. 2018, 42,776.
|
| [46] |
Johnson, F.E.; Hamilton, C.S. J. Am. Chem. Soc. 1949, 71,74.
|
| [47] |
Catalano, A.; Carocci, A.; Defrenza, I.; Muraglia, M.; Carrieri, A.; Bambeke, F.V.; Rosato, A.; Corbo, F.; Franchini, C. Eur. J. Med. Chem. 2013, 64,357.
|
| [48] |
Naeimi, H.; Heidarnezhad, A. Res. Chem. Intermed. 2016, 42,7855.
|
| [49] |
Bondock, S.; Fadaly, W.; Metwally, M.A. J. Sulfur Chem. 2009, 30,74.
|
| [50] |
Bhalerao, D.S.; Akamanchi, K.G. Synlett 2007,2952.
|
| [51] |
Piscitelli, F.; Ballatore, C.; Smith III, A.B. Bioorg. Med. Chem. Lett. 2010, 20,644.
pmid: 19954974 |
| [52] |
Morofuji, T.; Shimizu, A.; Yoshida, J. Chem. Eur. J. 2015, 21,3211.
pmid: 25641711 |
| [53] |
Joyce, L.; Evindar, G.; Batey, R.A. Chem. Commun. 2004,446.
|
| [54] |
Wang, J.; Peng, F.; Jiang, J.; Lu, Z.; Wang, L.; Bai, J.; Pan, Y. Tetrahedron Lett. 2008, 49,467.
|
| [55] |
Saha, P.; Ramana, T.; Purkait, N.; Ali, M.A.; Paul, R.; Punniyamurthy, T. J. Org. Chem. 2009, 74,8719.
|
| [56] |
Feng, E.; Huang, H.; Zhou, Y.; Ye, D.; Jiang, H.; Liu, H. J. Comb. Chem. 2010, 12,422.
|
| [57] |
Wang, R.; Yang, W.-j.; Yue, Liang., Zeng, H.-y.; Synlett 2012,1643.
|
| [58] |
Cheng, Y.; Peng, Q.; Fan, W.; Li, P. J. Org. Chem. 2014, 79,5812.
|
| [59] |
Aelterman, W.; Lang, Y.; Willemsens, B.; Vervest, I.; Leurs, S.; Knaeo, F.D. Org. Process Res. Dev. 2001, 5,467.
|
| [60] |
Inamoto, K.; Hasegawa, C.; Hiroya, K.; Doi, T. Org. Lett. 2008, 10,5147.
|
| [61] |
Joyce, L.L.; Batey, R.A. Org. Lett. 2009, 11,2792.
|
| [62] |
Inamoto, K.; Hasegawa, C.; Kawasaki, J.; Hiroya, K.; Doi, T. Adv. Synth. Catal. 2010, 352,2643.
|
| [63] |
Wang, H.; Wang, L.; Shang, J.; Li, X.; Wang, H.; Gui, J.; Lei, A. Chem. Commun. 2012, 48,76.
|
| [64] |
Shen, C.; Xia, H.; Yan, H.; Chen, X.; Ranjit, S.; Xie, X.; Tan, D.; Lee, R.; Yang, Y.; Xing, B.; Huang, K.-W.; Zhang, P.; Liu, X. Chem. Sci. 2012, 3,2388.
|
| [65] |
Wang, J.; Zong, Y.; Zhang, X.; Gao, Y.; Li, Z.; Yue, G.; Quan, Z.; Wang, X. Synlett 2014, 25,2143.
|
| [66] |
Nahakpam, L.; Chingakham, B.S.; Laitonjam, W.S. J. Heterocycl. Chem. 2015, 52,267.
|
| [67] |
Gogoi, P.; Paul, B.; Hazarika, S.; Barman, P. RSC Adv. 2015, 5,57433.
|
| [68] |
Srivastava, V.; Singh, P.K.; Singh, P.P. Croat. Chem. Acta 2015, 88,227.
|
| [69] |
Sharma, S.; Pathare, R.S.; Maurya, A.K.; Gopal, K.; Roy, T.K.; Sawant, D.M.; Pardasani, R.T. Org. Lett. 2016, 18,356.
pmid: 26761401 |
| [70] |
Zeng, M.-T.; Xu, W.; Liu, M.; Liu, X.; Chang, C.-Z.; Zhu, H.; Dong, Z.-B. SynOpen 2017, 1,1.
|
| [71] |
Benedí, C.; Bravo, F.; Uriz, P.; Fernández, E.; Claver, C.; Castillόn, S. Tetrahedron Lett. 2003, 44,6073.
|
| [72] |
Kobayashi, K.; Kobayashi, A.; Murahashi, K.; Fukamachi, S. Heterocycles 2011, 83,2589.
|
| [73] |
Sahoo, S.K.; Banerjee, A.; Chakraborty, S.; Patel, B.K. ACS Catal. 2012, 2,544.
|
| [74] |
Rout, S.K.; Guin, S.; Nath, J.; Patel, B.K. Green Chem. 2012, 14,2491.
|
| [75] |
Sahoo, S.K.; Khatun, N.; Gogoi, A.; Deb, A.; Patel, B.K. RSC Adv. 2013, 3,438.
|
| [76] |
Pinapati, S.; Mandapati, U.; Tamminana, R.; Rudraraju, R. ChemistrySelect 2019, 4,254.
|
| [77] |
Ding, Q.; He, X.; Wu, J. J. Comb. Chem. 2009, 11,587.
pmid: 19449803 |
| [78] |
Shen, G.; Lv, X.; Bao, W. Eur. J. Org. Chem. 2009,5897.
|
| [79] |
Qiu, J.-W.; Zhang, X.-G.; Tang, R.-Y.; Zhong, P.; Li, J.-H. Adv. Synth. Catal. 2009, 351,2319.
|
| [80] |
Guo, Y.-J.; Tang, R.-Y.; Zhong, P.; Li, J.-H. Tetrahedron Lett. 2010, 51,649.
|
| [81] |
Ding, Q.; Cao, B.; Liu, X.; Zong, Z.; Peng, Y.-Y. Green Chem. 2010, 12,1607.
|
| [82] |
Canon, R.; Ramόn, D.J.; Yus, M. J. Org. Chem. 2011, 76,654.
pmid: 21175155 |
| [83] |
Sun, Y.-L.; Zhang, Y.; Cui, X.-H.; Wang, W. Adv. Synth. Catal. 2011, 353,1174.
|
| [84] |
Yang, J.; Li, P.; Wang, L. Tetrahedron 2011, 67,5543.
|
| [85] |
Xiao, R.; Hao, W.; Ai, J.; Cai, M.-Z. J. Organomet. Chem. 2012, 705,44.
|
| [86] |
Wang, R.; Chen, Z.; Yue, L.; Pan, W.; Zhao, J.-J. Tetrahedron Lett. 2012, 53,4529.
|
| [87] |
Zhang, W.; Yue, Y.; Yu, D.; Song, L.; Xu, Y.-Y.; Tian, Y.-J.; Guo, Y.-J. Adv. Synth. Catal. 2012, 354,2283.
|
| [88] |
Khatum, N.; Jamir, L.; Ganesh, M.; Patel, B.K. RSC Adv. 2012, 2,11557.
|
| [89] |
Yao, R.; Liu, H.; Wu, Y.; Cai, M. Appl. Organomet. Chem. 2013, 27,109.
|
| [90] |
Zhao, N.; Liu, L.; Wang, F.; Li, J.; Zhang, W. Adv. Synth. Catal. 2014, 356,2575.
|
| [91] |
Liu, J.; Zhang, X.; Yang, J.; Wang, L. Appl. Organomet. Chem. 2014, 28,198.
|
| [92] |
Gaddam, S.; Kasireddy, H.R.; Konkala, K.; Katla, R.; Durga, N.Y. V. Chin. Chem. Lett. 2014, 25,732.
|
| [93] |
Azizi, K.; Karimi, M.; Heydari, A. Tetrahedron Lett. 2015, 56,812.
|
| [94] |
Moghadam, F.K.; Jarrah, N.; Mashayekh-Salehi, A.; Ghanbaripour, R. Synlett 2016, 27,1665.
|
| [95] |
Chen, L.; Huang, B.; Nie, Q.; Cai, M. Appl. Organomet. Chem. 2016, 30,446.
|
| [96] |
Mishra, N.; Singh, A.S.; Agrahari, A.K.; Singh, S.K.; Singh, M.; Tiwari, V.K. ACS Comb. Sci. 2019, 21,389.
|
| [97] |
Le, Z.-G.; Xu, J.-P.; Rao, H.-Y.; Ying, M. J. Heterocycl. Chem. 2006, 43,1123.
|
| [98] |
Banerjee, A.; Santra, S.K.; Rout, S.K.; Patel, B.K. Tetrahedron 2013, 69,9096.
|
| [99] |
Xu, Y.; Li, B.; Zhang, X.; Fan, X. J. Org. Chem. 2017, 82,9637.
|
| [100] |
Wang, P.; Tang, S.; Lei, A. Green Chem. 2017, 19,2092.
|
| [101] |
He, Y.; Li, J.; Luo, S.; Huang, J.; Zhu, Q. Chem. Commun. 2016, 52,8444.
|
| [102] |
Mirza, B.; Mirzazadeh, R.; Zeeb, M. J. Chem. Res. 2013, 37,778.
|
| [103] |
Yao, G.; Wang, B.-F.; Yang, S.; Zhang, Z.-X.; Xu, H.-H.; Tang, R.-Y. RSC Adv. 2019, 9,3403.
|
| [104] |
El-Sharief, A.M. Sh.; Ammar, Y.A.; Zahran, M.A.; Sabet, H.K. Phosphorus, Sulfur Silicon Relat. Elem. 2004, 179,267.
|
| [105] |
Cee, V.J.; Downing, N.S. Tetrahedron Lett. 2006, 47,3747.
|
| [106] |
Yella, R.; Patel, B.K. J. Comb. Chem. 2010, 12,754.
doi: 10.1021/cc100124q pmid: 20718465 |
| [107] |
Wang, W.; Zhong, W.; Zhou, R.; Yu, J.; Dai, J.; Ding, Q.; Peng, Y. Heterocycles 2010, 81,2841.
|
| [108] |
Ding, Q.; Cao, B.; Yang, Q.; Liu, X.; Peng, Y. Phosphorus, Sulfur Silicon Relat. Elem. 2011, 186,1782.
|
| [109] |
Zhang, X.; Jia, X.; Wang, J.; Fan, X. Green Chem. 2011, 13,413.
|
| [110] |
Sharma, R.; Bala, M.; Verma, P.K.; Singh, B. Synth. Commun. 2015, 45,2106.
|
| [111] |
Xie, Y.; Zhang, F.; Chen, X.; Li, J. Heterocycles 2010, 81,2087.
|
| [112] |
Fajkusova, D.; Pesko, M.; Keltosova, S.; Guo, J.; Oktabec, Z.; Vejsova, M.; Kollar, P.; Coffey, A.; Csollei, J.; Kralova, K.; Jampilek, J. Bioorg. Med. Chem. 2012, 20,7059.
|
| [113] |
Fajkusova, D.; Pazdera, P. Synthesis 2008,1297.
|
| [114] |
El-Faham, A.; Chebbo, M.; Abdul-Ghani, M.; Younes, G. J. Heterocycl. Chem. 2006, 43,599.
|
| [115] |
Vlaar, T.; Cioc, R.C.; Mampuys, P.; Maes, B.U. W.; Orru, R.V. A.; Ruijter, E. Angew. Chem. Int. Ed. 2012, 51,13058.
|
| [116] |
Zhu, T.-H.; Wang, S.-Y.; Wang, G.-N.; Ji, S.-J. Chem.-Eur. J. 2013, 19,5850.
pmid: 23526676 |
| [117] |
Wang, G.-N.; Zhu, T.-H.; Wang, S.-Y.; Wei, T.-Q.; Ji, S.-J. Tetrahedron 2014, 70,8079.
|
| [118] |
Zhu, T.-H.; Xu, X.-P.; Cao, J.-J.; Wei, T.-Q.; Wang, S.-Y.; Ji, S.-J. Adv. Synth. Catal. 2014, 356,509.
|
| [119] |
Dumonteil, G.; Hiebel, M.-A.; Scherrmann, M.-C.; Berteina-Raboin, S. RSC Adv. 2016, 6,73517.
|
| [120] |
Min, H, Ziao, G.; Liu, W.; Liang, Y. Org. Biomol. Chem. 2016, 14,11088.
doi: 10.1039/c6ob02413k pmid: 27872926 |
| [121] |
Pi, S.-S.; Zhang, X.-G.; Tang, R.-Y.; Li, J.-H. Synlett 2009,3032.
|
| [122] |
Katari, N.K.; Venkatanarayana, M.; Srinivas, K. J. Chem. Sci. 2015, 127,447.
|
| [123] |
Xu, W.; Zeng, M.-T.; Liu, M.; Liu, S.-S.; Li, Y.-S.; Dong, Z.-B. Synthesis 2017, 49,3084.
|
| [124] |
Xu, W.; Zeng, M.-T.; Liu, M.; Liu, X.; Chang, C.-Z.; Zhu, H.; Dong, Z.-B. J. Sulfur Chem. 2017, 38,644.
|
| [125] |
Ma, D.; Lu, X.; Shi, L.; Zhang, H.; Jiang, Y.; Liu, X. Angew. Chem. Int. Ed. 2011, 50,1118.
|
| [126] |
Satish, G.; Reddy, K.H. V.; Ramesh, K.; Karnakar, K.; Nageswar, Y.V. D. Tetrahedron Lett. 2012, 53,2518.
doi: 10.1016/j.tetlet.2012.03.012 |
| [127] |
Zhang, Z.; Wang, F.-J.; Wu, H.-H.; Tan, Y.-J. Chem. Lett. 2015, 44,440.
|
| [128] |
Takagi, K. Chem. Lett. 1986, 15,265.
|
| [129] |
Monguchi, D.; Fujiwara, T.; Furukawa, H.; Mori, A. Org. Lett. 2009, 11,1607.
pmid: 19254040 |
| [130] |
Tanba, S.; Fujiwara, T.; Monguchi, D.; Mori, A. J. Phys.: Conf. Ser. 2010, 232,012010.
|
| [131] |
Wang, Q.; Schreiber, S.L. Org. Lett. 2009, 11,5178.
|
| [132] |
Cho, S.H.; Kim, J.Y.; Lee, S.Y.; Chang, S. Angew. Chem. Int. Ed. 2009, 48,9127.
|
| [133] |
Kim, J.Y.; Cho, S.H.; Joseph, J.; Chang, S. Angew. Chem. Int. Ed. 2010, 49,9899.
|
| [134] |
Mitsuda, S.; Fujiwara, T.; Kimigafukuro, K.; Monguchi, D.; Mori, A. Tetrahedron 2012, 68,3585.
|
| [135] |
Satish, G.; Reddy, H.V.; Anil, B.S. P.; Shankar, J.; Kumar, R.U.; Nageswar, Y.V. D. Tetrahedron Lett. 2014, 55,5533.
|
| [136] |
Walczyński, K.; Guryn, R.; Zuiderveld, O.P.; Timmerman, H. Farmaco 1999, 54,684.
|
| [137] |
Charles, M.D.; Schultz, P.; Buchwald, S.L. Org. Lett. 2005, 7,3965.
pmid: 16119943 |
| [138] |
Motiwala, H.F.; Kumar, R.; Chakraborti, A.K. Aust. J. Chem. 2007, 60,369.
|
| [139] |
Ramana, M.M. V.; Sharma, M.R. J. Chem. Pharm. Res. 2013, 5,122.
|
| [140] |
Toulot, S.; Heinrich, T.; Leroux, F.R. Adv. Synth. Catal. 2013,355 , 3263.
|
| [141] |
Reddy, K.H. V.; Kumar, B.S. P.; Reddy, V.P.; Kumar, R.U.; Nageswar, Y.V. D. RSC Adv. 2014, 4,45579.
|
| [142] |
Kumar, R.U.; Reddy, H.V.; Kumar, B. S., P.A.; Satish, G.; Reddy, V.P.; Nageswar, Y.V. D. Tetrahedron Lett. 2016, 57,637.
|
| [143] |
Zhu, Y.-Q.; Zhang, R.; Sang, W.; Wang, H.-J.; Wu, Y.; Yu, B.-Y.; Zhang, J.-C.; Cheng, H.; Chen, C. Org. Chem. Front. 2020, 7,1981.
|
| [144] |
Yin, J.; Zhao, M.M.; Huffman, M.A.; Mcnamara, J.M. Org. Lett. 2002, 4,3481.
pmid: 12323049 |
| [145] |
Shen, Q.; Ogata, T.; Hartwig, J.F. J. Am. Chem. Soc. 2008, 130,6586.
pmid: 18444639 |
| [146] |
Murthy, S.N.; Madhav, B.; Reddy, V.P.; Nageswar, Y.V. D. Adv. Synth. Catal. 2010, 352,3241.
|
| [147] |
Heo, Y.; Hyun, D.; Kumar, M.R.; Jung, H.M.; Lee, S. Tetrahedron Lett. 2012, 53,6657.
|
| [148] |
McGowan, M.A.; Henderson, J.L.; Buchwald, S.L. Org. Lett. 2012, 14,1432.
pmid: 22394197 |
| [149] |
Das, R.; Mandal, M.; Chakraborty, D. Asian J. Org. Chem. 2013, 2,579.
|
| [150] |
Wang, D.; Kuang, D.; Zhang, F.; Liu, Y.; Ning, S. Tetrahedron Lett. 2014, 55,7121.
|
| [151] |
Wang, P.; Li, J.; Jiang, X.; Liu, Z.; Ye, N.; Xu, Y.; Yang, G.; Xu, Y.; Zhang, A. Tetrahedron 2014, 70,5666.
|
| [152] |
Majumder, A.; Gupta, R.; Mandal, M.; Babu, M.; Chakraborty, D. J. Organomet. Chem. 2015, 781,23.
|
| [153] |
Rao, D.N.; Rasheed, SK.; Aravinda, S.; Vishwakarma, R.A.; Das, P. RSC Adv. 2013, 3,11472.
|
| [154] |
Han, Y.; Zhang, M.; Zhang, Y.-Q.; Zhang, Z.-H. Green Chem. 2018, 20,4891.
|
| [155] |
Chand, S.; Kumar, S.; Singh, R.; Singh, K.N. ChemistrySelect 2019, 4,718.
|
| [156] |
Moser, D.; Duan, Y.; Wang, F.; Ma, Y.; O’Neil, M.J.; Cornella, J. Angew. Chem. Int. Ed. 2018, 57,11035.
|
| [157] |
Saima; Kumar, L.; Lavekar, A. G.; Sharma, T.; Shamsuzzama; Equbal, D.; Siddiqi, M. I.; Sinha, A. K.; Nazir, A. ChemistrySelect 2018, 3,3539.
|
| [158] |
Lee, E.; An, Y.; Kwon, J.; Kim, K.I.; Jeon, R. Bioorg. Med. Chem. 2017, 25,3617.
|
| [159] |
Ji, Z.; Zhou, F.; Wei, S. Bioorg. Med. Chem. Lett. 2015, 25,4065.
pmid: 26318996 |
| [160] |
Ismail, M.M. F.; Abdulwahab, H.G.; Nossier, E.S.; Menofy, N.G. E.; Abdelkhalek, B.A. Bioorg. Chem. 2020, 94,103437.
doi: 10.1016/j.bioorg.2019.103437 pmid: 31812260 |
| [161] |
Jian, R.-K.; Wang, P.; Duan, W.; Wang, J.; Zheng, X.; Weng, J. Ind. Eng. Chem. Res. 2016, 55,11520.
|
| [162] |
Padilla-Martínez, I.I.; González-Encarnación, J.M.; García-Báez, E.V.; Cruz, A.; Ramos-Organillo, Á.A. Molecules 2019, 24,3391.
|
| [163] |
Stewart, G.W.; Baxter, C.A.; Cleator, E.; Sheen, F.J. J. Org. Chem. 2009, 74,3229.
pmid: 19317430 |
| [164] |
Zeng, W.; Dang, P.; Zhang, X.; Liang, Y.; Peng, C. RSC Adv. 2014, 4,31003.
|
| [165] |
Boddapati, S.N. M.; Kurmarayuni, C.M.; Mutchu, B.R.; Tamminana, R.; Bollikolla, H.B. Org. Biomol. Chem. 2018, 16,8267.
|
| [166] |
Castanheiro, T.; Suffert, J.; Gulea, M.; Donnard, M. Org. Lett. 2016, 18,2588.
pmid: 27192105 |
| [167] |
Kumar, D.; Mishra, B.B.; Tiwari, V.K. J. Org. Chem. 2014, 79,251.
pmid: 24313219 |
| [168] |
Zhao, J.; Huang, H.; Wu, W.; Chen, H.; Jiang, H. Org. Lett. 2013, 15,2604.
pmid: 23662734 |
| [1] | 冯康博, 陈炯, 古双喜, 王海峰, 陈芬儿. 全连续流反应技术在药物合成中的新进展(2019~2022)[J]. 有机化学, 2024, 44(2): 378-397. |
| [2] | 李鹏辉, 谢青洋, 万福贤, 张元红, 姜林. 含环丙基的新型取代嘧啶-5-甲酰胺的合成及杀菌活性研究[J]. 有机化学, 2024, 44(2): 650-656. |
| [3] | 邹发凯, 王能中, 姚辉, 王慧, 刘明国, 黄年玉. 1β-/3R-芳基硫代糖的区域与立体选择性合成[J]. 有机化学, 2024, 44(2): 593-604. |
| [4] | 李路瑶, 贺忠文, 张振国, 贾振华, 罗德平. 三芳基碳正离子在有机合成中的应用[J]. 有机化学, 2024, 44(2): 421-437. |
| [5] | 梅青刚, 李清寒. 可见光促进C(3)(杂)芳硫基吲哚化合物的合成研究进展[J]. 有机化学, 2024, 44(2): 398-408. |
| [6] | 赵茜帆, 陈永正, 张世明. 碳基非金属催化剂在有机合成领域的应用及机理研究[J]. 有机化学, 2024, 44(1): 137-147. |
| [7] | 陈珊, 陈志林, 胡琼, 蒙艳双, 黄悦, 陶萍芳, 卢丽如, 黄国保. 含双硫脲基团分子钳在非极性溶剂中识别中性分子[J]. 有机化学, 2024, 44(1): 277-281. |
| [8] | 王化坤, 任晓龙, 宣宜宁. 卤盐催化的α,β-环氧羧酸酯与异氰酸酯[3+2]环加成反应研究[J]. 有机化学, 2024, 44(1): 251-258. |
| [9] | 金玉坤, 任保轶, 梁福顺. 可见光介导的三氟甲基的选择性C-F键断裂及其在偕二氟类化合物合成中的应用[J]. 有机化学, 2024, 44(1): 85-110. |
| [10] | 马翠云, 罗海澜, 张福华, 郭丹, 陈树兴, 王飞. 3-Pyrrolyl BODIPY的绿色生物合成、光物理性质及应用研究[J]. 有机化学, 2024, 44(1): 216-223. |
| [11] | 王博珍, 张婕, 粘春惠, 金茗茗, 孔苗苗, 李物兰, 何文斐, 吴建章. 含有3,4-二氯苯基的酰胺类化合物的合成及抗肿瘤活性研究[J]. 有机化学, 2024, 44(1): 232-241. |
| [12] | 黄志友, 杨平, 何波, 欧文霞, 袁思雨. 吗啉磺酰胺化合物的设计、合成及其抑制大豆萌芽活性的研究[J]. 有机化学, 2024, 44(1): 309-315. |
| [13] | 杨维清, 葛宴兵, 陈元元, 刘萍, 付海燕, 马梦林. 1,8-萘酰亚胺衍生物的设计、合成及其对半胱氨酸的识别研究[J]. 有机化学, 2024, 44(1): 180-194. |
| [14] | 于士航, 刘嘉威, 安碧玉, 边庆花, 王敏, 钟江春. 黑腹尼虎天牛接触性信息素的不对称合成[J]. 有机化学, 2024, 44(1): 301-308. |
| [15] | 李阳, 袁锦鼎, 赵頔. 低共熔溶剂1,3-二甲基脲/L-(+)-酒石酸中(E)-2-苯乙烯基喹啉-3-羧酸类衍生物的绿色合成[J]. 有机化学, 2023, 43(9): 3268-3276. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||