有机化学 ›› 2021, Vol. 41 ›› Issue (8): 3106-3115.DOI: 10.6023/cjoc202102035 上一篇 下一篇
研究论文
葛雅欣a, 闫芹芹a, 田云飞b,*( ), 王海军a, 张春芳a,*(
), 王海军a, 张春芳a,*( ), 李泽江a,*(
), 李泽江a,*( )
)
收稿日期:2021-02-20
修回日期:2021-04-17
发布日期:2021-06-02
通讯作者:
田云飞, 张春芳, 李泽江
基金资助:
Yaxin Gea, Qinqin Yana, Yunfei Tianb( ), Haijun Wanga, Chunfang Zhanga(
), Haijun Wanga, Chunfang Zhanga( ), Zejiang Lia(
), Zejiang Lia( )
)
Received:2021-02-20
Revised:2021-04-17
Published:2021-06-02
Contact:
Yunfei Tian, Chunfang Zhang, Zejiang Li
Supported by:文章分享

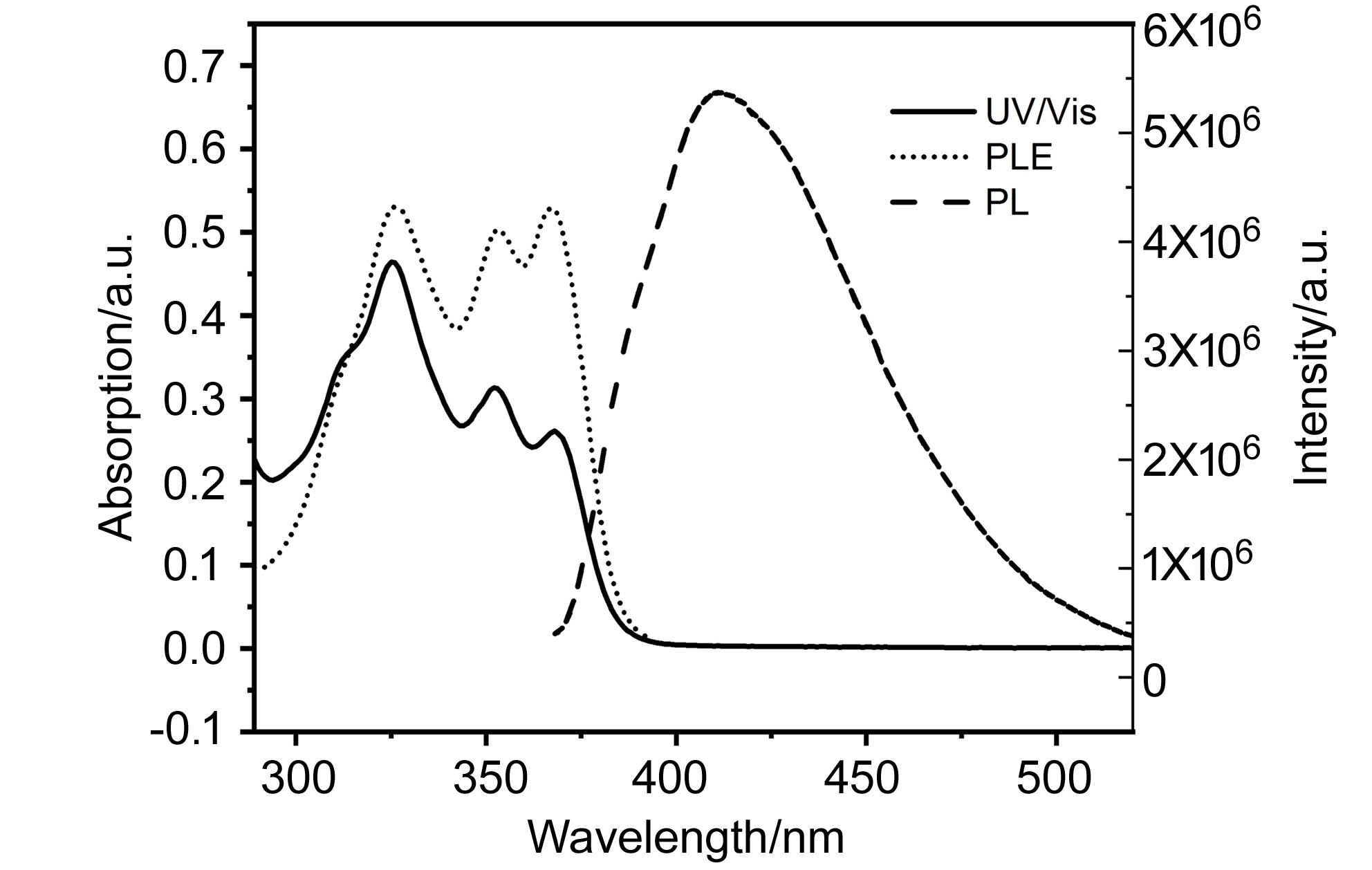
发展了一类自由基引发不饱和酰胺与多氯甲烷的串联合环反应, 该体系提供了一种可选择性地生成多氯甲基取代的异喹啉酮或二氢异喹啉酮的方法. 同时, 在该工作中开展了机理研究和光学实验.
葛雅欣, 闫芹芹, 田云飞, 王海军, 张春芳, 李泽江. 无金属参与下不饱和酰胺与多氯甲烷的串联环化反应[J]. 有机化学, 2021, 41(8): 3106-3115.
Yaxin Ge, Qinqin Yan, Yunfei Tian, Haijun Wang, Chunfang Zhang, Zejiang Li. Metal-Free-Involved Cascade Cyclization of Unsaturated Amides with Polychloromethanes[J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3106-3115.
| Entry | Catalyst (mol%) | Initiator (equiv.) | Solvent (mL) | Yieldb/% |
|---|---|---|---|---|
| 1 | KI (5) | DTBP (5.0) | Chloroform (7) | 37 |
| 2 | KI (5) | DCP (5.0) | Chloroform (7) | 60 |
| 3 | KI (5) | TBCP (5.0) | Chloroform (7) | 49 |
| 4 | KI (5) | TBPB (5.0) | Chloroform (7) | 39 |
| 5 | KI (5) | DCP (3.0) | Chloroform (7) | 25 |
| 6 | KI (5) | DCP (4.0) | Chloroform (7) | 32 |
| 7 | KI (5) | DCP (6.0) | Chloroform (7) | 38 |
| 8 | KI (10) | DCP (5.0) | Chloroform (7) | 33 |
| 9 | NIS (5) | DCP (5.0) | Chloroform (7) | 46 |
| 10 | NaI (5) | DCP (5.0) | Chloroform (7) | 50 |
| 11 | KI (5) | DCP (5.0) | Chloroform (3) | 44 |
| 12 | KI (5) | DCP (5.0) | Chloroform (5) | 38 |
| 13c | KI (5) | DCP (5.0) | Chloroform (7) | 26 |
| 14d | KI (5) | DCP (5.0) | Chloroform (7) | Trace |
| 15e | KI (5) | DCP (5.0) | Chloroform (7) | Trace |
| Entry | Catalyst (mol%) | Initiator (equiv.) | Solvent (mL) | Yieldb/% |
|---|---|---|---|---|
| 1 | KI (5) | DTBP (5.0) | Chloroform (7) | 37 |
| 2 | KI (5) | DCP (5.0) | Chloroform (7) | 60 |
| 3 | KI (5) | TBCP (5.0) | Chloroform (7) | 49 |
| 4 | KI (5) | TBPB (5.0) | Chloroform (7) | 39 |
| 5 | KI (5) | DCP (3.0) | Chloroform (7) | 25 |
| 6 | KI (5) | DCP (4.0) | Chloroform (7) | 32 |
| 7 | KI (5) | DCP (6.0) | Chloroform (7) | 38 |
| 8 | KI (10) | DCP (5.0) | Chloroform (7) | 33 |
| 9 | NIS (5) | DCP (5.0) | Chloroform (7) | 46 |
| 10 | NaI (5) | DCP (5.0) | Chloroform (7) | 50 |
| 11 | KI (5) | DCP (5.0) | Chloroform (3) | 44 |
| 12 | KI (5) | DCP (5.0) | Chloroform (5) | 38 |
| 13c | KI (5) | DCP (5.0) | Chloroform (7) | 26 |
| 14d | KI (5) | DCP (5.0) | Chloroform (7) | Trace |
| 15e | KI (5) | DCP (5.0) | Chloroform (7) | Trace |

| [1] |
For selected recent reviews, see:
pmid: 16895332 |
|
(a) Gribble, G. W. Acc. Chem. Res. 1998, 31, 141.
doi: 10.1021/ar9701777 pmid: 16895332 |
|
|
(b) Vaillancourt, F. H.; Yeh, E.; Vosburg, D. A.; Garneau-Tsodikova, S.; Walsh, C. T. Chem. Rev. 2006, 106, 3364.
pmid: 16895332 |
|
|
(c) Paul, C.; Pohnert, G. Nat. Prod. Rep. 2011, 28, 186.
doi: 10.1039/C0NP00043D pmid: 16895332 |
|
|
(d) Latham, J.; Brandenburger, E.; Shepherd, S. A.; Menon, B. R. K.; Micklefield, J. Chem. Rev. 2018, 118, 232.
doi: 10.1021/acs.chemrev.7b00032 pmid: 16895332 |
|
| [2] |
For selected recent reviews, see:
pmid: 18459798 |
|
(a) Sitachitta, N.; Rossi, J.; Roberts, M. A.; Gerwick, W. H.; Fletcher, M. D.; Willis, C. L. J. Am. Chem. Soc. 1998, 120, 7131.
doi: 10.1021/ja9811389 pmid: 18459798 |
|
|
(b) Ardá, A.; Soengas, R. G.; Nieto, M. I.; Jiménez, C.; Rodríguez, J. Org. Lett. 2008, 10, 2175.
doi: 10.1021/ol800551g pmid: 18459798 |
|
|
(c) Nguyen, V.-A.; Willis, C. L.; Gerwick, W. H. Chem. Commun. 2001, 1934.
pmid: 18459798 |
|
| [3] |
For selected recent references, see:
|
|
(a) Butler, A.; Walker, J. V. Chem. Rev. 1993, 93, 1937.
doi: 10.1021/cr00021a014 |
|
|
(b) Morimoto, H.; Lu, G.; Aoyama, N.; Matsunaga, S.; Shibasaki, M. J. Am. Chem. Soc. 2007, 129, 9588.
doi: 10.1021/ja073285p |
|
|
(c) Miles, Z. D.; Diethelm, S.; Pepper, H. P.; Huang, D. M.; George, J. H.; Moore, B. S. Nat. Chem. 2017, 9, 1235.
doi: 10.1038/nchem.2829 |
|
| [4] |
For selected recent reviews, see:
pmid: 16551084 |
|
(a) Vaillancourt, F. H.; Yeh, E.; Vosburg, D. A.; O'Connor, S. E.; Walsh, C. T. Nature 2005, 436, 1191.
doi: 10.1038/nature03797 pmid: 16551084 |
|
|
(b) Galonić, D. P.; Vaillancourt, F. H.; Walsh, C. T. J. Am. Chem. Soc. 2006, 128, 3900.
pmid: 16551084 |
|
| [5] |
For selected recent reviews, see:
|
|
(a) Beaumont, S.; Ilardi, E. A.; Monroe, L. R.; Zakarian, A. J. Am. Chem. Soc. 2010, 132, 1482.
doi: 10.1021/ja910154f |
|
|
(b) Gu, Z. H.; Herrmann, A. T.; Zakarian, A. Angew. Chem., Int. Ed. 2011, 50, 7136.
doi: 10.1002/anie.v50.31 |
|
| [6] |
Lu, M. Z.; Loh, T.-P. Org. Lett. 2014, 16, 4698.
doi: 10.1021/ol502411c |
| [7] |
Li, X.; Xu, J.; Gao, Y.; Fang, H.; Tang, G.; Zhao, Y. J. Org. Chem. 2015, 80, 2621.
doi: 10.1021/jo502777b |
| [8] |
For selected recent reviews on cyclization reactions, see:
|
|
(a) Liu, Y.; Zhang, J.-L.; Song, R.-J.; Li, J.-H. Org. Chem. Front. 2014, 1, 1289.
doi: 10.1039/C4QO00251B |
|
|
(b) Liang, Y.; Lv, G.; Ouyang, X.; Song, R.-J.; Li, J.-H. Adv. Synth. Catal. 2020, 363, 290.
doi: 10.1002/adsc.v363.2 |
|
|
(c) Tian, Y.; Liu, Z.-Q. RSC Adv. 2014, 4, 64855.
doi: 10.1039/C4RA12032A |
|
|
(d) Pan, C.; Wu, C.; Yuan, C.; Yu, J.-T. Tetrahedron Lett. 2020, 61, 151499.
doi: 10.1016/j.tetlet.2019.151499 |
|
|
(e) Huang, G.; Yu, J.-T.; Pan, C.-D. Adv. Synth. Catal. 2020, 363, 305.
doi: 10.1002/adsc.v363.2 |
|
|
(l) Li, W.; Sun, Y.; Yao, Y.; Xu, Y.; Li, P.; Liu, Y.; Liang, D. Chin. J. Org. Chem. 2019, 39, 1727. (in Chinese)
doi: 10.6023/cjoc201901047 |
|
|
(李文兰, 孙一茼, 姚永超, 许颖, 李鹏, 刘颖杰, 梁德强, 有机化学, 2019, 39, 1727.)
doi: 10.6023/cjoc201901047 |
|
| [9] |
For selected recent reviews, see:
|
|
(a) Pettit, G. R.; Gaddamidi, V.; Cragg, G. M.; Herald, D. L.; Sagawa, Y. J. Chem. Soc., Chem. Commun. 1984, 1692.
|
|
|
(b) Pettit, G. R.; Meng, Y.; Herald, D. L.; Graham, K. A. N.; Pettit, R. K.; Doubek, D. L. J. Nat. Prod. 2003, 66, 1065.
doi: 10.1021/np0300986 |
|
|
(c) Krane, B. D.; Shamma, M. J. Nat. Prod. 1982, 45, 377.
doi: 10.1021/np50022a001 |
|
|
(d) Cheng, K.; Rahier, N. J.; Eisenhauer, B. M.; Gao, R.; Thomas, S. J.; Hecht, S. M. J. Am. Chem. Soc. 2005, 127, 838.
doi: 10.1021/ja0442769 |
|
| [10] |
Scott, J. D.; Williams, R. M. Chem. Rev. 2002, 102, 1669.
pmid: 11996547 |
| [11] |
For selected recent reviews, see:
|
|
(a) Kajita, Y.; Matsubara, S.; Kurahashi, T. J. Am. Chem. Soc. 2008, 130, 6058.
doi: 10.1021/ja7114426 |
|
|
(b) Ackermann, L.; Lygin, A. V.; Hofmann, N. Angew. Chem., Int. Ed. 2011, 50, 6379.
doi: 10.1002/anie.201101943 |
|
|
(c) Antczak, M. I.; Ready, J. M. Chem. Sci. 2012, 3, 1450.
doi: 10.1039/c2sc20102j |
|
|
(d) Upadhyay, N. S.; Thorat, V. H.; Sato, R.; Annamalai, P.; Chuang, S.-C.; Cheng, C.-H. Green Chem. 2017, 19, 3219.
doi: 10.1039/C7GC01221G |
|
|
(e) Hédouin, J.; Carpentier, V.; Renard, R. M. Q.; Schneider, C.; Gillaizeau, I.; Hoarau, C. J. Org. Chem. 2019, 84, 10535.
doi: 10.1021/acs.joc.9b01408 |
|
|
(f) Fang, Z.; Wang, Y.; Wang, Y. Org. Lett. 2019, 21, 434.
doi: 10.1021/acs.orglett.8b03614 |
|
|
(g) Lee, J.; Kim, H. Y.; Oh, K. Org. Lett. 2020, 22, 474.
doi: 10.1021/acs.orglett.9b04233 |
|
| [12] |
For selected recent reviews, see:
|
|
(a) Azizian, J.; Mohammadi, A. A.; Karimi, A. R.; Mohammadizadeh, M. R. J. Organomet. Chem. 2005, 70, 350.
|
|
|
(b) Vara, Y.; Bello, T.; Aldaba, E.; Arrieta, A.; Pizarro, J. L.; Arriortua, M. I.; Lopez, X.; Cossio, F. P. Org. Lett. 2008, 10, 4759.
doi: 10.1021/ol801757r |
|
|
(c) Wang, L.; Liu, J.; Tian, H.; Qian, C.; Sun, J. Adv. Synth. Catal. 2005, 347, 689.
doi: 10.1002/(ISSN)1615-4169 |
|
| [13] |
For selected recent reviews, see:
pmid: 21275421 |
|
(a) Tang, Q.; Xia, D.; Jin, X.; Zhang, Q.; Sun, X.-Q.; Wang, C. J. Am. Chem. Soc. 2013, 135, 4628.
doi: 10.1021/ja400020e pmid: 21275421 |
|
|
(b) Rakshit, S.; Grohmann, C.; Besset, T.; Glorius, F. J. Am. Chem. Soc. 2011, 133, 2350.
doi: 10.1021/ja109676d pmid: 21275421 |
|
|
(c) Li, B.; Ma, J.; Wang, N.; Feng, H.; Xu, S.; Wang, B. Org. Lett. 2012, 14, 736.
doi: 10.1021/ol2032575 pmid: 21275421 |
|
|
(d) Zhang, S.-S.; Wu, J.-Q.; Liu, X.; Wang, H. ACS Catal. 2015, 5, 210.
doi: 10.1021/cs501601c pmid: 21275421 |
|
| [14] |
For selected recent radical cyclization reactions, see:
pmid: 26011072 |
|
(a) Zhou, W.; Ni, S.; Mei, H.; Han, J.; Pan, Y. Org. Lett. 2015, 17, 2724.
doi: 10.1021/acs.orglett.5b01140 pmid: 26011072 |
|
|
(b) Xia, D.; Li, Y.; Miao, T.; Li, P.; Wang, L. Chem. Commun. 2016, 52, 11559.
doi: 10.1039/C6CC04983D pmid: 26011072 |
|
|
(c) Wang, J.; Sun, K.; Chen, X.; Chen, T.; Liu, Y.; Qu, L.; Zhao, Y.; Yu, B. Org. Lett. 2019, 21, 1863.
doi: 10.1021/acs.orglett.9b00465 pmid: 26011072 |
|
|
(d) Zou, L.; Li, P.; Wang, B.; Wang, L. Green Chem. 2019, 21, 3362.
doi: 10.1039/C9GC00938H pmid: 26011072 |
|
|
(e) Pan, C.-D.; Wang, Y.; Wu, C.; Yu, J.-T. Catal. Commun. 2019, 131, 105802.
doi: 10.1016/j.catcom.2019.105802 pmid: 26011072 |
|
|
(f) Liu, X.; Qian, P.; Wang, Y.; Pan, Y. Org. Chem. Front. 2017, 4, 2370.
doi: 10.1039/C7QO00677B pmid: 26011072 |
|
|
(g) Xu, Z.-Q.; Wang, C.; Li, L.; Duan, L.; Li, Y.-M. J. Org. Chem. 2018, 83, 9718.
doi: 10.1021/acs.joc.8b01242 pmid: 26011072 |
|
| [15] |
(a) Li, Z.-J.; Cui, X.; Niu, L.; Ren, Y.; Bian, M.; Yang, X.; Yang, B.; Yan, Q.; Zhao, J. Adv. Synth. Catal. 2017, 359, 246.
doi: 10.1002/adsc.v359.2 |
|
(b) Zhang, R.; Yu, H.; Li, Z.-J.; Yan, Q.; Li, P.; Wu, J.; Qi, J.; Jiang, M.; Sun, L. Adv. Synth. Catal. 2018, 360, 1384.
doi: 10.1002/adsc.v360.7 |
|
|
(c) Ge, Y.; Tian, Y.; Wu, J.; Yan, Q.; Zheng, L.; Ren, Y.; Zhao, J.; Li, Z.-J. Chem. Commun. 2020, 56, 12656.
doi: 10.1039/D0CC05213B |
|
|
(d) Wu, J.; Zong, Y.; Zhao, C.; Yan, Q.; Sun, L.; Li, Y.; Zhao, J.; Ge, Y.; Li, Z.-J. Org. Biomol. Chem. 2019, 17, 794.
doi: 10.1039/C8OB02964D |
|
| [16] |
Kundu, G.; Sperger, T.; Rissanen, K.; Schoenebeck, F. Angew. Chem., Int. Ed. 2020, 59, 21930.
doi: 10.1002/anie.v59.49 |
| [1] | 黄芬, 罗维纬, 周俊. 基于C—H键断裂的多氯烷基化反应研究进展[J]. 有机化学, 2023, 43(7): 2368-2390. |
| [2] | 田钰, 张娟, 高文超, 常宏宏. 二甲亚砜作为甲基化试剂在有机合成中的应用[J]. 有机化学, 2023, 43(7): 2391-2406. |
| [3] | 赵金晓, 魏彤辉, 柯森, 李毅. 可见光催化合成二氟烷基取代的多环吲哚化合物[J]. 有机化学, 2023, 43(3): 1102-1114. |
| [4] | 杜昌远, 唐裕才, 段京林, 杨碧玉, 何宇鹏, 周谦, 刘学文. 可见光促进有机染料催化2-芳基吲哚自由基烷氧羰基化反应研究[J]. 有机化学, 2023, 43(12): 4268-4276. |
| [5] | 胡朝明, 吴纪红, 吴晶晶, 吴范宏. 直接三氟甲硒基化反应研究进展[J]. 有机化学, 2023, 43(1): 36-56. |
| [6] | 宇世伟, 陈兆华, 陈淇, 林舒婷, 何金萍, 陶冠燊, 汪朝阳. 硫代磺酸酯的合成与应用研究进展[J]. 有机化学, 2022, 42(8): 2322-2330. |
| [7] | 龚诚, 唐剑, 徐飞, 李鹏杰, 王泽田, 张玉敏, 余国贤, 王亮. 近年来过渡金属催化吡啶酮/异喹啉酮的C—H活化反应研究进展[J]. 有机化学, 2022, 42(7): 1925-1949. |
| [8] | 张力之, 廖永剑, 陈宁, 黄磊, 周敏. 叔丁醇钾促进的环化和偶联反应[J]. 有机化学, 2022, 42(7): 1950-1959. |
| [9] | 李亚东, 吴鹏举, 杨志勇. 可见光催化苯并噁唑与α-酮酸合成芳基苯并噁唑[J]. 有机化学, 2022, 42(6): 1770-1777. |
| [10] | 乐柏佟, 吴新鑫, 朱晨. 烯基自由基参与的分子内氢原子转移反应的新进展[J]. 有机化学, 2022, 42(2): 458-470. |
| [11] | 孙亚敏, 李锡勇, 袁金伟, 余加琳, 刘帅楠. 温和条件下以芳基胺为原料CuI催化下区域选择性合成3-芳基香豆素[J]. 有机化学, 2022, 42(2): 631-640. |
| [12] | 许耀辉, 吴镇, 吴新鑫, 朱晨. 无过渡金属参与的醚、醛和酰胺C—H键自由基炔基化和烯丙基化反应[J]. 有机化学, 2022, 42(12): 4340-4349. |
| [13] | 徐浩, 张杰, 左峻泽, 王丰晓, 吕健, 混旭, 杨道山. 硫鎓盐在可见光催化构建C—C键及C—杂原子键中的应用进展[J]. 有机化学, 2022, 42(12): 4037-4059. |
| [14] | 肖潜, 佟庆笑, 钟建基. 基于自由基串联环化反应合成苯并吖庚因衍生物的研究进展[J]. 有机化学, 2022, 42(12): 3979-3994. |
| [15] | 高盼盼, 肖文精, 陈加荣. 可见光促进的烯烃合成研究进展[J]. 有机化学, 2022, 42(12): 3923-3943. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||