有机化学 ›› 2022, Vol. 42 ›› Issue (4): 1146-1162.DOI: 10.6023/cjoc202107062 上一篇 下一篇
研究论文
王玉斌a, 郭成b, 陶晟a, 刘纪昌a,c, 赵基钢a,c, 刘宁a,*( ), 代斌a,*(
), 代斌a,*( )
)
收稿日期:2021-07-29
修回日期:2021-09-29
发布日期:2022-05-10
通讯作者:
刘宁, 代斌
基金资助:
Yubin Wanga, Cheng Guob, Sheng Taoa, Jichang Liua,c, Jigang Zhaoa,c, Ning Liua( ), Bin Daia(
), Bin Daia( )
)
Received:2021-07-29
Revised:2021-09-29
Published:2022-05-10
Contact:
Ning Liu, Bin Dai
Supported by:文章分享
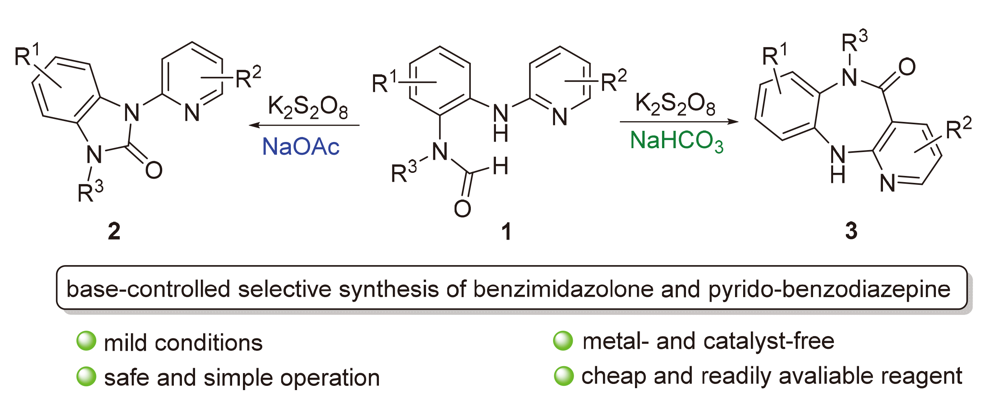
发展了一种碱控制选择性合成苯并咪唑酮和吡啶并苯二氮䓬衍生物的方法. 该方法以N-烷基-N-(2-(吡啶-2-基氨基)苯基)甲酰胺类化合物为原料, 以K2S2O8为氧化剂, 当选用NaOAc为碱时, 高选择性地得到了一系列苯并咪唑酮衍生物; 当选用NaHCO3为碱时, 得到了一系列吡啶并苯二氮䓬衍生物. 通过自由基捕捉实验的研究, 提出了相应可能的反应机理. 苯二氮䓬的克级放大实验和官能化衍生化实验说明该方法具有一定应用前景.
王玉斌, 郭成, 陶晟, 刘纪昌, 赵基钢, 刘宁, 代斌. 碱性调控的选择性: 通过N-烷基-N-(2-(吡啶-2-基氨基)苯基)甲酰胺合成苯并咪唑酮和苯二氮䓬类化合物[J]. 有机化学, 2022, 42(4): 1146-1162.
Yubin Wang, Cheng Guo, Sheng Tao, Jichang Liu, Jigang Zhao, Ning Liu, Bin Dai. Basicity-Tuned Selectivity: Synthesis of Benzimidazolone and Benzodiazepine from N-Alkyl-N-(2-(pyridin-2-ylamino)-phenyl)formamides[J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1146-1162.
| Entry | 2a, Condition Aa | 2a, Condition Bb | 3a, Condition Cc |
|---|---|---|---|
| 1 | 2a, 80%; (3a, 0) | 2a, 82% | 3a, 68%; (2a, trace) |
| 2 | No K2S2O8, 2a, 0 No base, 2a, 8% | No PhI(OAc)2, 2a, 0 | No K2S2O8, 3a, 0 No base, 3a, trace |
| 3 | LiOt-Bu instead of NaOAc, 2a, 12% Na2CO3 instead of NaOAc, 2a, 10% KHCO3 instead of NaOAc, 2a, trace K2CO3 instead of NaOAc, 2a, 11% | — | LiOt-Bu instead of NaHCO3, 3a, 26% Na2CO3 instead of NaHCO3, 3a, 29% KHCO3 instead of NaHCO3, 3a, 10% K2CO3 instead of NaHCO3, 3a, 22% |
| 4 | DCE instead of MeCN, 2a, 30% DMSO instead of MeCN, 2a, 26% 1,4-Dioxane instead of MeCN, 2a, 35% MeCN/H2O (V∶V=1∶1), 2a, 36% | DCE instead of MeCN, 2a, 69% DMSO instead of MeCN, 2a, 80% 1,4-Dioxane instead of MeCN, 2a, 77% MeCN/H2O (V∶V=1∶1), 2a, 40% | DCE instead of MeCN, 3a, 40% DMSO instead of MeCN, 3a, 15% 1,4-Dioxane instead of MeCN, 3a, 10% MeCN/H2O (V∶V=1∶1), 3a, 26% |
| 5 | Na2S2O8 instead of K2S2O8, 2a, 39% (NH4)2S2O8 instead of K2S2O8, 2a, 45% DDQ instead of K2S2O8, 2a, 0 TBHP instead of K2S2O8, 2a, 0 | Na2S2O8 instead of PhI(OAc)2, 2a, 0 (NH4)2S2O8 instead of PhI(OAc)2, 2a, 0 DDQ instead of PhI(OAc)2, 2a, 0 TBHP instead of PhI(OAc)2, 2a, 0 | Na2S2O8 instead of K2S2O8, 3a, 38% (NH4)2S2O8 instead of K2S2O8, 3a, 32% DDQ instead of K2S2O8, 3a, 0 TBHP instead of K2S2O8, 3a, trace |
| 6 | K2S2O8 (1.0 equiv.), 2a, 65% K2S2O8 (1.5 equiv.), 2a, 80% K2S2O8 (2.0 equiv.), 2a, 79% | PhI(OAc)2 (1.0 equiv.), 2a, 47% PhI(OAc)2 (1.5 equiv.), 2a, 82% PhI(OAc)2 (2.0 equiv.), 2a, 84% | K2S2O8 (1.0 equiv.), 3a, 36% K2S2O8 (1.5 equiv.), 3a, 61% K2S2O8 (2.0 equiv.), 3a, 23% |
| 7 | NaOAc (1.5 equiv.), 2a, 69% NaOAc (2.0 equiv.), 2a, 80% NaOAc (3.0 equiv.), 2a, 78% | — | NaHCO3 (1.5 equiv.), 3a, 61% NaHCO3 (2.0 equiv.), 3a, 69% NaHCO3 (3.0 equiv.), 3a, 67% |
| 8 | 100 ℃ instead of 80 ℃, 2a, 80% 50 ℃ instead of 80 ℃, 2a, 58% | 50 ℃ instead of r.t., 2a, 85% | 100 ℃ instead of 80 ℃, 3a, 65% 50 ℃ instead of 80 ℃, 3a, 47% |
| 9 | — | — | Air, 3a, 59% |
| 10 | 48 h, 2a, 80% | 5 h, 2a, 80% | 48 h, 3a, 65% |
| Entry | 2a, Condition Aa | 2a, Condition Bb | 3a, Condition Cc |
|---|---|---|---|
| 1 | 2a, 80%; (3a, 0) | 2a, 82% | 3a, 68%; (2a, trace) |
| 2 | No K2S2O8, 2a, 0 No base, 2a, 8% | No PhI(OAc)2, 2a, 0 | No K2S2O8, 3a, 0 No base, 3a, trace |
| 3 | LiOt-Bu instead of NaOAc, 2a, 12% Na2CO3 instead of NaOAc, 2a, 10% KHCO3 instead of NaOAc, 2a, trace K2CO3 instead of NaOAc, 2a, 11% | — | LiOt-Bu instead of NaHCO3, 3a, 26% Na2CO3 instead of NaHCO3, 3a, 29% KHCO3 instead of NaHCO3, 3a, 10% K2CO3 instead of NaHCO3, 3a, 22% |
| 4 | DCE instead of MeCN, 2a, 30% DMSO instead of MeCN, 2a, 26% 1,4-Dioxane instead of MeCN, 2a, 35% MeCN/H2O (V∶V=1∶1), 2a, 36% | DCE instead of MeCN, 2a, 69% DMSO instead of MeCN, 2a, 80% 1,4-Dioxane instead of MeCN, 2a, 77% MeCN/H2O (V∶V=1∶1), 2a, 40% | DCE instead of MeCN, 3a, 40% DMSO instead of MeCN, 3a, 15% 1,4-Dioxane instead of MeCN, 3a, 10% MeCN/H2O (V∶V=1∶1), 3a, 26% |
| 5 | Na2S2O8 instead of K2S2O8, 2a, 39% (NH4)2S2O8 instead of K2S2O8, 2a, 45% DDQ instead of K2S2O8, 2a, 0 TBHP instead of K2S2O8, 2a, 0 | Na2S2O8 instead of PhI(OAc)2, 2a, 0 (NH4)2S2O8 instead of PhI(OAc)2, 2a, 0 DDQ instead of PhI(OAc)2, 2a, 0 TBHP instead of PhI(OAc)2, 2a, 0 | Na2S2O8 instead of K2S2O8, 3a, 38% (NH4)2S2O8 instead of K2S2O8, 3a, 32% DDQ instead of K2S2O8, 3a, 0 TBHP instead of K2S2O8, 3a, trace |
| 6 | K2S2O8 (1.0 equiv.), 2a, 65% K2S2O8 (1.5 equiv.), 2a, 80% K2S2O8 (2.0 equiv.), 2a, 79% | PhI(OAc)2 (1.0 equiv.), 2a, 47% PhI(OAc)2 (1.5 equiv.), 2a, 82% PhI(OAc)2 (2.0 equiv.), 2a, 84% | K2S2O8 (1.0 equiv.), 3a, 36% K2S2O8 (1.5 equiv.), 3a, 61% K2S2O8 (2.0 equiv.), 3a, 23% |
| 7 | NaOAc (1.5 equiv.), 2a, 69% NaOAc (2.0 equiv.), 2a, 80% NaOAc (3.0 equiv.), 2a, 78% | — | NaHCO3 (1.5 equiv.), 3a, 61% NaHCO3 (2.0 equiv.), 3a, 69% NaHCO3 (3.0 equiv.), 3a, 67% |
| 8 | 100 ℃ instead of 80 ℃, 2a, 80% 50 ℃ instead of 80 ℃, 2a, 58% | 50 ℃ instead of r.t., 2a, 85% | 100 ℃ instead of 80 ℃, 3a, 65% 50 ℃ instead of 80 ℃, 3a, 47% |
| 9 | — | — | Air, 3a, 59% |
| 10 | 48 h, 2a, 80% | 5 h, 2a, 80% | 48 h, 3a, 65% |
| [1] |
(a) Poupaert, J.; Carato, P.; Colacino, E. Curr. Med. Chem. 2005, 12, 877.
pmid: 19788239 |
|
(b) Welsch, M. E.; Snyder, S. A.; Stockwell, B. R. Curr. Opin. Chem. Biol. 2010, 14, 347.
doi: 10.1016/j.cbpa.2010.02.018 pmid: 19788239 |
|
|
(c) Lo, H. Y.; Nemoto, P. A.; Kim, J. M.; Hao, M.-H.; Qian, K. C.; Farrow, N. A.; Albaugh, D. R.; Fowler, D. M.; Schneiderman, R. D.; Michael August, E.; Martin, L.; Hill-Drzewi, M.; Pullen, S. S.; Takahashi, H.; De Lombaert, S. Bioorg. Med. Chem. Lett. 2011, 21, 4533.
doi: 10.1016/j.bmcl.2011.05.126 pmid: 19788239 |
|
|
(d) Filippakopoulos, P.; Qi, J.; Picaud, S.; Shen, Y.; Smith, W. B.; Fedorov, O.; Morse, E. M.; Keates, T.; Hickman, T. T.; Felletar, I.; Philpott, M.; Munro, S.; Mckeown, M. R.; Wang, Y.; Christie, A. L.; West, N.; Cameron, M. J.; Schwartz, B.; Heightman, T. D.; La Thangue, N.; French, C. A.; Wiest, O.; Kung, A. L.; Knapp, S.; Bradner, J. E. Nature 2010, 468, 1067.
doi: 10.1038/nature09504 pmid: 19788239 |
|
|
(e) Králová, P.; Maloň, M.; Soural, M. ACS Comb. Sci. 2017, 19, 770.
doi: 10.1021/acscombsci.7b00134 pmid: 19788239 |
|
|
(f) Kundu, P.; Mondal, A.; Das, B.; Chowdhury, C. Adv. Synth. Catal. 2015, 357, 3737.
doi: 10.1002/adsc.201500661 pmid: 19788239 |
|
|
(g) Carlier, P. R.; Zhao, H. W.; Macquarrie-Hunter, S. L.; Deguzman, J. C.; Hsu, D. C. J. Am. Chem. Soc. 2006, 128, 15215.
pmid: 19788239 |
|
|
(h) Al-Tel, T. H.; Al-Qawasmeh, R. A.; Schmidt, M. F.; Al-Aboudi, A.; Rao, S. N.; Sabri, S. S.; Voelter, W. J. Med. Chem. 2009, 52, 6484.
doi: 10.1021/jm9008482 pmid: 19788239 |
|
|
(i) Wang, Y. Y.; Ling, B. P.; Liu, P.; Bi, S. W. Organometallics 2018, 37, 3035.
doi: 10.1021/acs.organomet.8b00406 pmid: 19788239 |
|
| [2] |
Meanwell, N. A.; Sit, S.-Y.; Gao, J.; Boissard, C. G.; Lum-Ragan, J.; Dworetzky, S. I.; Gribkoff, V. K. Bioorg. Med. Chem. Lett. 1996, 6, 1641.
|
| [3] |
(a) P. Barot,, K.; Nikolova, S.; Ivanov, I.; D. Ghate,, M. Mini-Rev. Med. Chem. 2013, 13, 1421.
doi: 10.2174/13895575113139990072 |
|
(b) Yu, K.-L.; Sin, N.; Civiello, R. L.; Wang, X. A.; Combrink, K. D.; Gulgeze, H. B.; Venables, B. L.; Wright, J. J. K.; Dalterio, R. A.; Zadjura, L.; Marino, A.; Dando, S.; D’arienzo, C.; Kadow, K. F.; Cianci, C. W.; Li, Z.; Clarke, J.; Genovesi, E. V.; Medina, I.; Lamb, L.; Colonno, R. J.; Yang, Z.; Krystal, M.; Meanwell, N. A. Bioorg. Med. Chem. Lett. 2007, 17, 895.
doi: 10.1016/j.bmcl.2006.11.063 |
|
|
(c) Yu, K.-L.; Wang, X. A.; Civiello, R. L.; Trehan, A. K.; Pearce, B. C.; Yin, Z. W.; Combrink, K. D.; Gulgeze, H. B.; Zhang, Y.; Kadow, K. F.; Cianci, C. W.; Clarke, J.; Genovesi, E. V.; Medina, I.; Lamb, L.; Wyde, P. R.; Krystal, M.; Meanwell, N. A. Bioorg. Med. Chem. Lett. 2006, 16, 1115.
doi: 10.1016/j.bmcl.2005.11.109 |
|
| [4] |
(a) Monforte, A.-M.; Logoteta, P.; Ferro, S.; Luca, L. D.; Iraci, N.; Maga, G.; Clercq, E. D.; Pannecouque, C.; Chimirri, A. Biorg. Med. Chem. 2009, 17, 5962.
doi: 10.1016/j.bmc.2009.06.068 |
|
(b) Monforte, A.-M.; Logoteta, P.; Luca, L. D.; Iraci, N.; Ferro, S.; Maga, G.; De Clercq, E.; Pannecouque, C.; Chimirri, A. Biorg. Med. Chem. 2010, 18, 1702.
doi: 10.1016/j.bmc.2009.12.059 |
|
| [5] |
Gustin, D. J.; Sehon, C. A.; Wei, J. M.; Cai, H.; Meduna, S. P.; Khatuya, H.; Sun, S. Q.; Gu, Y.; Jiang, W.; Thurmond, R. L.; Karlsson, L.; Edwards, J. P. Bioorg. Med. Chem. Lett. 2005, 15, 1687.
pmid: 15745822 |
| [6] |
Kawamoto, H.; Nakashima, H.; Kato, T.; Arai, S.; Kamata, K.; Iwasawa, Y. Tetrahedron 2001, 57, 981.
doi: 10.1016/S0040-4020(00)01064-4 |
| [7] |
Diao, X. Q.; Wang, Y. J.; Jiang, Y. W.; Ma, D. W. J. Org. Chem. 2009, 74, 7974.
doi: 10.1021/jo9017183 |
| [8] |
Mclaughlin, M.; Palucki, M.; Davies, I. W. Org. Lett. 2006, 8, 3311.
doi: 10.1021/ol061233j |
| [9] |
Beyer, A.; Reucher, C. M. M.; Bolm, C. Org. Lett. 2011, 13, 2876.
doi: 10.1021/ol2008878 |
| [10] |
(a) Meng, Y. G.; Wang, B. N.; Ren, L. N.; Zhao, Q. L.; Yu, W. Q.; Chang, J. B. New J. Chem. 2018, 42, 13790.
doi: 10.1039/C8NJ03166E |
|
(b) Yu, J. P.; Gao, C.; Song, Z. X.; Yang, H. J.; Fu, H. Eur. J. Org. Chem. 2015, 2015, 5869.
doi: 10.1002/ejoc.201500726 |
|
| [11] |
Youn, S. W.; Kim, Y. H. Org. Lett. 2016, 18, 6140.
pmid: 27934371 |
| [12] |
Xu, F.; Long, H.; Song, J. S.; Xu, H.-C. Angew. Chem., Int. Ed. 2019, 58, 9017.
doi: 10.1002/anie.201904931 |
| [13] |
Li, J.-S.; Yang, P.-P.; Xie, X.-Y.; Jiang, S.; Tao, L.; Li, Z.-W.; Lu, C.-H.; Liu, W.-D. Adv. Synth. Catal. 2020, 362, 1977.
doi: 10.1002/adsc.202000198 |
| [14] |
(a) Lima, H. M.; Lovely, C. J. Org. Lett. 2011, 13, 5736.
doi: 10.1021/ol2022438 |
|
(b) Hamm, M. L.; Billig, K. Org. Biomol. Chem. 2006, 4, 4068.
doi: 10.1039/B612597B |
|
|
(c) Bon, R. S.; Sprenkels, N. E.; Koningstein, M. M.; Schmitz, R. F.; De Kanter, F. J. J.; Dömling, A.; Groen, M. B.; Orru, R. V. A. Org. Biomol. Chem. 2008, 6, 130.
doi: 10.1039/B713065A |
|
| [15] |
Li, D. Z.; Ollevier, T. Org. Lett. 2019, 21, 3572.
doi: 10.1021/acs.orglett.9b00973 |
| [16] |
Chernyshev, V. M.; Khazipov, O. V.; Shevchenko, M. A.; Chernenko, A. Y.; Astakhov, A. V.; Eremin, D. B.; Pasyukov, D. V.; Kashin, A. S.; Ananikov, V. P. Chem. Sci. 2018, 9, 5564.
doi: 10.1039/c8sc01353e pmid: 30061988 |
| [17] |
Ruiz, J.; Mesa, A. F. Chem.-Eur. J. 2014, 20, 102.
doi: 10.1002/chem.201303773 |
| [18] |
Fader, L. D.; Bethell, R.; Bonneau, P.; Bös, M.; Bousquet, Y.; Cordingley, M. G.; Coulombe, R.; Deroy, P.; Faucher, A.-M.; Gagnon, A.; Goudreau, N.; Grand-Maître, C.; Guse, I.; Hucke, O.; Kawai, S. H.; Lacoste, J.-E.; Landry, S.; Lemke, C. T.; Malenfant, E.; Mason, S.; Morin, S.; O’meara, J.; Simoneau, B.; Titolo, S.; Yoakim, C. Bioorg. Med. Chem. Lett. 2011, 21, 398.
doi: 10.1016/j.bmcl.2010.10.131 pmid: 21087861 |
| [19] |
Eltze, M.; Mutschler, E.; Lambrecht, G. Eur. J. Pharmacol. 1992, 211, 283.
pmid: 1377628 |
| [20] |
Hargrave, K. D.; Proudfoot, J. R.; Grozinger, K. G.; Cullen, E.; Kapadia, S. R.; Patel, U. R.; Fuchs, V. U.; Mauldin, S. C.; Vitous, J.; Behnke, M. L.; Klunder, J. M.; Pal, K.; Skiles, J. W.; Mcneil, D. W.; Rose, J. M.; Chow, G. C.; Skoog, M. T.; Wu, J. C.; Schmidt, G.; Engel, W. W.; Eberlein, W. G.; Saboe, T. D.; Campbell, S. J.; Rosenthal, A. S.; Adams, J. J. Med. Chem. 1991, 34, 2231.
pmid: 1712395 |
| [21] |
Pèpe, G.; Reboul, J.-P.; Oddon, Y. Eur. J. Med. Chem. 1989, 24, 1.
doi: 10.1016/0223-5234(89)90157-8 |
| [22] |
(a) Yuan, S.; Yue, Y.-L.; Zhang, D.-Q.; Zhang, J.-Y.; Yu, B.; Liu, H.-M. Chem. Commun. 2020, 56, 11461.
doi: 10.1039/D0CC04875E |
|
(b) Hwang, J.; Borgelt, L.; Wu, P. ACS Comb. Sci. 2020, 22, 495.
doi: 10.1021/acscombsci.0c00173 |
|
|
(c) Velasco-Rubio, Á.; Varela, J. A.; Saá, C. Adv. Synth. Catal. 2020, 362, 4861.
doi: 10.1002/adsc.202000808 |
|
|
(d) Dagar, A.; Kim, I. Org. Biomol. Chem. 2020, 18, 9836.
doi: 10.1039/D0OB02002H |
|
|
(e) Archer, G. A.; Sternbach, L. H. Chem. Rev. 1968, 68, 747.
doi: 10.1021/cr60256a004 |
|
| [23] |
(a) Chobanian, H. R.; Guo, Y.; Liu, P.; Lanza, T. J.; Chioda, M.; Chang, L.; Kelly, T. M.; Kan, Y. Q.; Palyha, O.; Guan, X.-M.; Marsh, D. J.; Metzger, J. M.; Raustad, K.; Wang, S.-P.; Strack, A. M.; Gorski, J. N.; Miller, R.; Pang, J. M.; Lyons, K.; Dragovic, J.; Ning, J. G.; Schafer, W. A.; Welch, C. J.; Gong, X. Y.; Gao, Y.-D.; Hornak, V.; Reitman, M. L.; Nargund, R. P.; Lin, L. S. Biorg. Med. Chem. 2012, 20, 2845.
doi: 10.1016/j.bmc.2012.03.029 |
|
(b) Liu, P.; Lanza, T. J.; Chioda, M.; Jones, C.; Chobanian, H. R.; Guo, Y.; Chang, L.; Kelly, T. M.; Kan, Y. Q.; Palyha, O.; Guan, X.-M.; Marsh, D. J.; Metzger, J. M.; Ramsay, K.; Wang, S.-P.; Strack, A. M.; Miller, R.; Pang, J. M.; Lyons, K.; Dragovic, J.; Ning, J. G.; Schafer, W. A.; Welch, C. J.; Gong, X. Y.; Gao, Y.-D.; Hornak, V.; Ball, R. G.; Tsou, N.; Reitman, M. L.; Wyvratt, M. J.; Nargund, R. P.; Lin, L. S. ACS Med. Chem. Lett. 2011, 2, 933.
doi: 10.1021/ml200207w |
|
|
(c) Matsufuji, T.; Shimada, K.; Kobayashi, S.; Ichikawa, M.; Kawamura, A.; Fujimoto, T.; Arita, T.; Hara, T.; Konishi, M.; Abe-Ohya, R.; Izumi, M.; Sogawa, Y.; Nagai, Y.; Yoshida, K.; Abe, Y.; Kimura, T.; Takahashi, H. Biorg. Med. Chem. 2015, 23, 89.
doi: 10.1016/j.bmc.2014.11.018 |
|
|
(d) Failli, A. A.; Shumsky, J. S.; Steffan, R. J.; Caggiano, T. J.; Williams, D. K.; Trybulski, E. J.; Ning, X.; Lock, Y.; Tanikella, T.; Hartmann, D.; Chan, P. S.; Park, C. H. Bioorg. Med. Chem. Lett. 2006, 16, 954.
doi: 10.1016/j.bmcl.2005.10.107 |
|
| [24] |
Maddess, M. L.; Li, C. M. Organometallics 2019, 38, 81.
doi: 10.1021/acs.organomet.8b00322 |
| [25] |
Novelli, F.; Sparatore, A.; Tasso, B.; Sparatore, F. Bioorg. Med. Chem. Lett. 1999, 9, 3031.
pmid: 10571170 |
| [26] |
Shi, F. Q.; Xu, X. X.; Zheng, L. Y.; Dang, Q.; Bai, X. J. Comb. Chem. 2008, 10, 158.
doi: 10.1021/cc7002039 |
| [27] |
(a) Zhu, H. Q.; Shang, T. B.; Lu, Z. H.; Luo, F.; Zhu, G. G. Chin. J. Org. Chem. 2020, 40, 3410. (in Chinese)
doi: 10.6023/cjoc202005066 |
|
( 朱海倩, 商甜波, 卢增辉, 罗芳, 朱钢国, 有机化学, 2020, 40, 3410.)
doi: 10.6023/cjoc202005066 |
|
|
(b) Tian, S. H.; Luo, T.; Zhu, Y. P.; Wan, J.-P. Chin. Chem. Lett. 2020, 31, 3073.
doi: 10.1016/j.cclet.2020.07.042 |
|
|
(c) Fu, L. Q.; Liu, Y. Y.; Wan, J.-P. Org. Lett. 2021, 23, 4363.
doi: 10.1021/acs.orglett.1c01301 |
|
|
(d) Yu, Q.; Liu, Y. Y.; Wan, J.-P. Org. Chem. Front. 2020, 7, 2770.
doi: 10.1039/D0QO00855A |
|
|
(e) Chen, Q. W.; Yang, Y. C.; Wang, X.; Zhang, Q.; Li, D. Chin. J. Org. Chem. 2020, 40, 451. (in Chinese)
|
|
|
( 陈倩雯, 杨耀成, 王霞, 张谦, 李栋, 有机化学, 2020, 40, 451.)
|
|
|
(f) Zhao, B. L.; Liu, Y. Y. Synthesis 2020, 52, 3211.
doi: 10.1055/s-0040-1707124 |
|
| [28] |
Tao, S.; Bu, Q. Q.; Shi, Q. Q.; Wei, D. H.; Dai, B.; Liu, N. Chem. Eur. J. 2020, 26, 3252.
doi: 10.1002/chem.201905828 |
| [29] |
(a) Karthikeyan, J.; Cheng, C.-H. Angew. Chem., Int. Ed. 2011, 50, 9880.
doi: 10.1002/anie.201104311 |
|
(b) Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147.
doi: 10.1021/cr900184e |
|
|
(c) Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094.
doi: 10.1002/anie.200806273 |
|
|
(d) Ackermann, L.; Vicente, R.; Kapdi, A. R. Angew. Chem., Int. Ed. 2009, 48, 9792.
doi: 10.1002/anie.200902996 |
|
|
(e) Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215.
doi: 10.1021/cr100280d |
|
|
(f) Liu, C.; Zhang, H.; Shi, W.; Lei, A. W. Chem. Rev. 2011, 111, 1780.
doi: 10.1021/cr100379j |
|
| [30] |
(a) Liu, Q.; Xie, G. Q.; Wang, Q.; Mo, Z. D.; Li, C.; Ding, S. J.; Wang, X. X. Tetrahedron 2019, 75, 130490.
doi: 10.1016/j.tet.2019.130490 |
|
(b) Minisci, F.; Citterio, A.; Giordano, C. Acc. Chem. Res. 1983, 16, 27.
doi: 10.1021/ar00085a005 |
|
|
(c) Yan, K.; Yang, D. S.; Wei, W.; Wang, F.; Shuai, Y. Y.; Li, Q. N.; Wang, H. J. Org. Chem. 2015, 80, 1550.
doi: 10.1021/jo502474z |
|
|
(d) Mandal, S.; Bera, T.; Dubey, G.; Saha, J.; Laha, J. K. ACS Catal. 2018, 8, 5085.
doi: 10.1021/acscatal.8b00743 |
|
|
(e) Zhang, Z.; Jia, C.; Kong, X.; Hussain, M.; Liu, Z.; Liang, W.; Jiang, L.; Jiang, H.; Ma, J. ACS Sustainable Chem. Eng. 2020, 8, 16463.
doi: 10.1021/acssuschemeng.0c05118 |
|
|
(f) Zhu, Y. C.; Huang, K. M.; Pan, J.; Qiu, X.; Luo, X.; Qin, Q. X.; Wei, J. L.; Wen, X. J.; Zhang, L. Z.; Jiao, N. Nat. Commun. 2018, 9, 2625.
doi: 10.1038/s41467-018-05014-w |
|
| [31] |
(a) Shi, Z. Z.; Glorius, F. Chem. Sci. 2013, 4, 829.
doi: 10.1039/C2SC21823B |
|
(b) Wang, X. Y.; Wang, S. C.; Gao, Y. M.; Sun, H.; Liang, X. A.; Bu, F. X.; Abdelilah, T.; Lei, A. W. Org. Lett. 2020, 22, 5429.
doi: 10.1021/acs.orglett.0c01796 |
|
|
(c) Chen, Z. C.; Zhang, H.; Zhou, S. F.; Cui, X. L. Chin. J. Org. Chem. 2020, 40, 3866. (in Chinese)
doi: 10.6023/cjoc202007005 |
|
|
( 陈志超, 张红, 周树锋, 崔秀灵, 有机化学, 2020, 40, 3866.)
doi: 10.6023/cjoc202007005 |
|
| [32] |
(a) Yi, H.; Zhang, G. T.; Wang, H. M.; Huang, Z. Y.; Wang, J.; Singh, A. K.; Lei, A. W. Chem. Rev. 2017, 117, 9016.
doi: 10.1021/acs.chemrev.6b00620 |
|
(b) Pan, G.-A.; Li, Y.; Li, J.-H. Org. Chem. Front. 2020, 7, 2486.
doi: 10.1039/D0QO00651C |
|
| [33] |
(a) Hollóczki, O.; Terleczky, P.; Szieberth, D.; Mourgas, G.; Gudat, D.; Nyulászi, L. J. Am. Chem. Soc. 2011, 133, 780.
doi: 10.1021/ja103578y pmid: 21174475 |
|
(b) Tao, S.; Guo, C.; Liu, N.; Dai, B. Organometallics 2017, 36, 4432.
doi: 10.1021/acs.organomet.7b00651 pmid: 21174475 |
|
| [34] |
(a) Khan, D.; Mukhtar, S.; Alsharif, M. A.; Alahmdi, M. I.; Ahmed, N. Tetrahedron Lett. 2017, 58, 3183.
doi: 10.1016/j.tetlet.2017.07.018 |
|
(b) Prasad, V.; Kale, R. R.; Mishra, B. B.; Kumar, D.; Tiwari, V. K. Org. Lett. 2012, 14, 2936.
doi: 10.1021/ol3012315 |
|
| [35] |
Chen, F.; Chen, D. T.; Shi, L.; Liu, N.; Dai, B. J. CO2 Util. 2016, 16, 391.
|
| [36] |
Wang, Y.-B.; Liu, B.-Y.; Bu, Q. Q.; Dai, B.; Liu, N. Adv. Synth. Catal. 2020, 362, 2930.
doi: 10.1002/adsc.202000186 |
| [1] | 李路瑶, 贺忠文, 张振国, 贾振华, 罗德平. 三芳基碳正离子在有机合成中的应用[J]. 有机化学, 2024, 44(2): 421-437. |
| [2] | 纪健, 刘进华, 管丛, 陈绪文, 赵芸, 刘顺英. 原位生成的磺酸催化N-磺酰基-1,2,3-三氮唑与醇偶联高区域选择性合成N2-取代1,2,3-三氮唑[J]. 有机化学, 2023, 43(3): 1168-1176. |
| [3] | 马彪, 章淼淼, 李占宇, 彭进松, 陈春霞. 无过渡金属催化的Suzuki-Type交叉偶联反应研究进展[J]. 有机化学, 2023, 43(2): 455-470. |
| [4] | 肖朵朵, 张建涛, 周鹏, 刘卫兵. 无金属条件下芳基酮与二甲亚砜的α-C(sp3)—H亚甲基化反应合成γ-酮亚砜[J]. 有机化学, 2023, 43(11): 3900-3906. |
| [5] | 李芳绍, 肖晶, 吴小芳, 王晓熠, 邓金凤, 唐子龙. 无金属条件下酰胺和酯参与的2-取代苯并噁唑衍生物的合成[J]. 有机化学, 2022, 42(6): 1778-1785. |
| [6] | 王馨瑶, 张晴晴, 刘书扬, 李敏, 李海芳, 段春迎, 金云鹤. 可见光诱导无金属条件下交叉脱氢偶联反应合成醌类苄基化衍生物[J]. 有机化学, 2022, 42(5): 1443-1452. |
| [7] | 张智鑫, 翟彤仪, 朱伯汉, 钱鹏程, 叶龙武. 无金属催化炔酰胺分子内[4+2]环化反应合成四氢吲哚衍生物[J]. 有机化学, 2022, 42(5): 1501-1508. |
| [8] | 许丽梅, 卢林燕, 蔡尽忠, 冯亚栋, 崔秀灵. 紫外光引发胺基苯酚与胺的自由基偶联反应构筑二胺基苯醌亚胺类化合物[J]. 有机化学, 2022, 42(4): 1210-1215. |
| [9] | 付拯江, 杨振江, 孙丽, 尹健, 伊学政, 蔡琥, 雷爱文. 无金属条件下亚磺酸钠与酚类化合物形成芳基磺酸酯的电化学合成反应[J]. 有机化学, 2022, 42(2): 600-606. |
| [10] | 陈任宏, 吴桂贞, 杨凯, 叶斌, 陈庆凤, 汪朝阳. 一锅法合成N-呋喃酮基磺酰腙类化合物[J]. 有机化学, 2021, 41(7): 2750-2759. |
| [11] | 郑茜茜, 刘云云, 万结平. 烯胺调控下和对甲苯磺酰叠氮在纯水介质中的无金属环化反应合成1,2,3-三氮唑[J]. 有机化学, 2021, 41(7): 2700-2706. |
| [12] | 谢坤宸, 江铭轩, 陈晓岚, 吕琪妍, 於兵. α-酮酸在无金属有机光合成中的应用[J]. 有机化学, 2021, 41(12): 4575-4587. |
| [13] | 何宇航, 杨慧, 高冬旭, 马嘉慧, 邵亚敏, 安光辉, 李光明. 可见光介导无金属的苯乙酸衍生物脱羧氘代[J]. 有机化学, 2021, 41(12): 4725-4731. |
| [14] | 石瑛, 秦富文, 王捷, 闫艳梅. 无金属参与的连续的Ugi三组分/炔烃-叠氮环加成反应一锅合成[1,2,3]三唑并[1,5- a]喹喔啉[J]. 有机化学, 2021, 41(1): 297-302. |
| [15] | 宋蒙蒙, 张志国, 郑丹, 李祥, 梁蕊, 赵旭娜, 时蕾, 张贵生. 高价碘试剂促进的N-芳基磺酰胺类化合物脱芳基反应[J]. 有机化学, 2020, 40(8): 2433-2441. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||