有机化学 ›› 2023, Vol. 43 ›› Issue (11): 3900-3906.DOI: 10.6023/cjoc202304011 上一篇 下一篇
研究论文
收稿日期:2023-04-08
修回日期:2023-06-25
发布日期:2023-07-12
基金资助:
Duoduo Xiao, Jiantao Zhang, Peng Zhou( ), Weibing Liu(
), Weibing Liu( )
)
Received:2023-04-08
Revised:2023-06-25
Published:2023-07-12
Contact:
E-mail: Supported by:文章分享
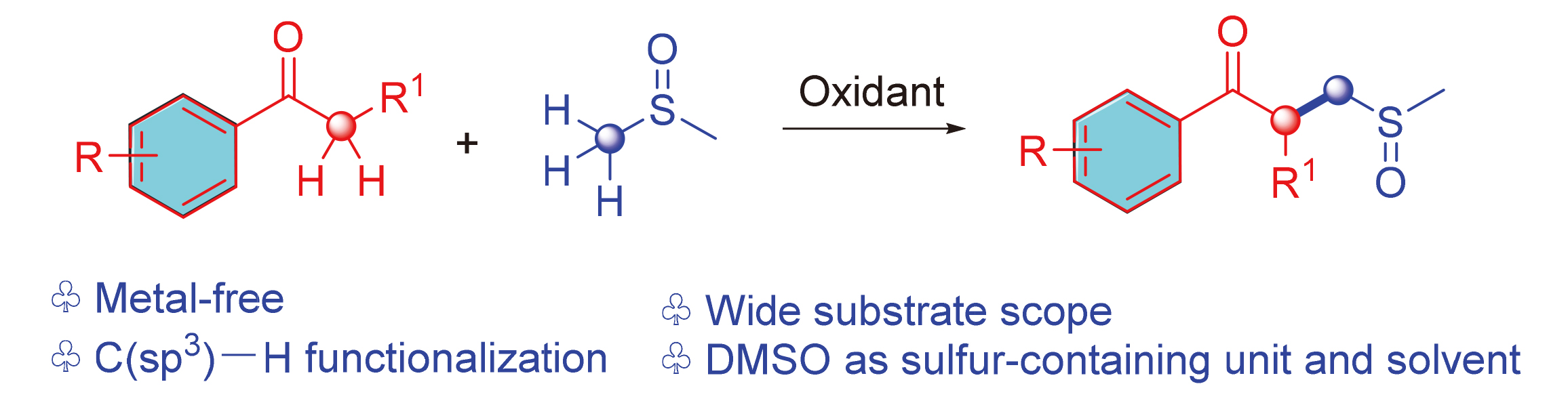
芳基酮通过C(sp3)—H键官能化与二甲亚砜发生直接的α-C(sp3)—H亚甲基化反应生成γ-酮亚砜, 该方法适用于各种芳基酮. 此外, 二甲基亚砜(DMSO)在反应中不仅用作溶剂, 而且用作含硫单元. 这种转化的实际价值在于γ-酮亚砜的高效和稳健的一锅合成法, 并在初步实验的基础上, 提出了一种可能的反应机理.
肖朵朵, 张建涛, 周鹏, 刘卫兵. 无金属条件下芳基酮与二甲亚砜的α-C(sp3)—H亚甲基化反应合成γ-酮亚砜[J]. 有机化学, 2023, 43(11): 3900-3906.
Duoduo Xiao, Jiantao Zhang, Peng Zhou, Weibing Liu. Metal-Free α-C(sp3)—H Methylenation of Aryl Ketones to Form γ-Keto Sulfoxides with Dimethyl Sulfoxide[J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3900-3906.
| Entry | Peroxide | T/℃ | t/h | Yieldb/% |
|---|---|---|---|---|
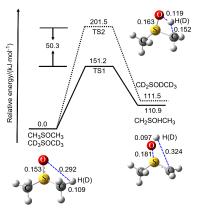
| 1 | (NH4)2S2O8 | 120 | 5 | 49 |
| 2 | (NH4)2S2O8 | 120 | 7 | 69 |
| 3 | (NH4)2S2O8 | 120 | 9 | 69 |
| 4 | (NH4)2S2O8 | 70 | 7 | — |
| 5 | (NH4)2S2O8 | r.t. | 7 | — |
| 6 | Benzoyl peroxide | 120 | 7 | — |
| 7 | Peroxide isobutylbenzene | 120 | 7 | — |
| 8 | K2S2O8 | 120 | 7 | 15 |
| 9 | DTBP | 120 | 7 | — |
| 10 | TBHP | 120 | 7 | — |
| 11c | (NH4)2S2O8 | 120 | 7 | 54 |
| 12d | (NH4)2S2O8 | 120 | 7 | 77 |
| 13d,e | (NH4)2S2O8 | 120 | 7 | 77 |
| 14d,f | (NH4)2S2O8 | 120 | 7 | 77 |
| Entry | Peroxide | T/℃ | t/h | Yieldb/% |
|---|---|---|---|---|
| 1 | (NH4)2S2O8 | 120 | 5 | 49 |
| 2 | (NH4)2S2O8 | 120 | 7 | 69 |
| 3 | (NH4)2S2O8 | 120 | 9 | 69 |
| 4 | (NH4)2S2O8 | 70 | 7 | — |
| 5 | (NH4)2S2O8 | r.t. | 7 | — |
| 6 | Benzoyl peroxide | 120 | 7 | — |
| 7 | Peroxide isobutylbenzene | 120 | 7 | — |
| 8 | K2S2O8 | 120 | 7 | 15 |
| 9 | DTBP | 120 | 7 | — |
| 10 | TBHP | 120 | 7 | — |
| 11c | (NH4)2S2O8 | 120 | 7 | 54 |
| 12d | (NH4)2S2O8 | 120 | 7 | 77 |
| 13d,e | (NH4)2S2O8 | 120 | 7 | 77 |
| 14d,f | (NH4)2S2O8 | 120 | 7 | 77 |

| [1] |
(a) Sang, P.; Chen, Q.; Wang, D.-Y.; Guo, W.; Fu, Y. Chem. Rev. 2023, 123, 1260.
|
|
(b) Engel, S.; Fritz, E. C.; Ravoo, B. J. Chem. Soc. Rev. 2017, 46, 2057.
doi: 10.1039/C7CS00023E |
|
|
(c) Otocka, S.; Kwiatkowska, M.; Madalińska, L.; Kiełbasiński, P. Chem. Rev. 2017, 117, 4147.
doi: 10.1021/acs.chemrev.6b00517 |
|
| [2] |
(a) Feng, M.; Mosiagin, I.; Kaiser, D.; Maryasin, B.; Maulide, N. J. Am. Chem. Soc. 2022, 144, 13044.
doi: 10.1021/jacs.2c05368 |
|
(b) Huang, X.; Maulide, N. J. Am. Chem. Soc. 2011, 133, 8510.
doi: 10.1021/ja2031882 |
|
| [3] |
(a) Bugaenko, D. I.; Volkov, A. A.; Andreychev, V. V.; Karchava, A. V. Org. Lett. 2023, 25, 272.
doi: 10.1021/acs.orglett.2c04143 pmid: 36594721 |
|
(b) Kaiser, D.; Klose, I.; Oost, R.; Neuhaus, J.; Maulide, N. Chem. Rev. 2019, 119, 8701.
doi: 10.1021/acs.chemrev.9b00111 pmid: 36594721 |
|
|
(c) Vizer, S. A.; Sycheva, E. S.; Al Quntar, A. A. A.; Kurmankulov, N. B.; Yerzhanov, K. B.; Dembitsky, V. M. Chem. Rev. 2015, 115, 1475.
doi: 10.1021/cr4001435 pmid: 36594721 |
|
| [4] |
(a) Wu, Y. H.; Wang, N. X.; Zhang, T.; Yan, Z.; Xu, B. C.; Inoa, J.; Xing, Y. Adv. Synth. Catal. 2019, 361, 3008.
doi: 10.1002/adsc.v361.12 |
|
(b) Song, S.; Huang, X.; Liang, Y. F.; Tang, C.; Li, X.; Jiao, N. Green Chem. 2015, 17, 2727.
doi: 10.1039/C5GC00184F |
|
| [5] |
(a) Yadav, P.; Awasthi, A; Gokulnath, S.; Tiwari, D. K. J. Org. Chem. 2021, 86, 2658.
doi: 10.1021/acs.joc.0c02696 |
|
(b) Phanindrudu, M.; Wakade, S. B.; Tiwari, D. K.; Likhar, P. R.; Tiwari, D. K. J. Org. Chem. 2018, 83, 9137.
doi: 10.1021/acs.joc.8b01204 |
|
|
(c) Wu, X.; Zhang, J.; Liu, S.; Gao, Q.; Wu, A. Adv. Synth. Catal. 2016, 358, 218.
doi: 10.1002/adsc.v358.2 |
|
| [6] |
(a) Tian, Y.; Zhang, J.; Gao, W.; Chang, H. Chin. J. Org. Chem. 2023, 43, 2391. (in Chinese)
doi: 10.6023/cjoc202211026 |
|
(田钰, 张娟, 高文超, 常宏宏, 有机化学, 2023, 43, 2391.)
doi: 10.6023/cjoc202211026 |
|
|
(b) Xu, C.; Jiang, S. F.; Wen, X. H.; Zhang, Q.; Zhou, Z. W.; Wu, Y. D.; Jia, F. C.; Wu, A. X. Adv. Synth. Catal. 2018, 360, 2267.
doi: 10.1002/adsc.v360.12 |
|
| [7] |
(a) Wang, M.; Tang, B. C.; Ma, J. T.; Wang, Z. X.; Xiang, J. C.; Wu, Y. D.; Wang, J. G.; Wu, A. X. Org. Biomol. Chem. 2019, 17, 1535.
doi: 10.1039/C8OB02994F |
|
(b) Liu, Y.; Hu, Y.; Cao, Z.; Zhan, X.; Luo, W.; Liu, Q.; Guo, C. Adv. Synth. Catal. 2019, 361, 1084.
doi: 10.1002/adsc.v361.5 |
|
| [8] |
(a) Jiang, Y.; Loh, T. P. Chem. Sci. 2014, 5, 4939.
doi: 10.1039/C4SC01901F |
|
(b) Yuan, G.; Zheng, J.; Gao, X.; Li, X.; Huang, L.; Chen, H.; Jiang, H. Chem. Commun. 2012, 48, 7513.
doi: 10.1039/c2cc32964f |
|
| [9] |
(a) Lin, Z.; Huang, L.; Yuan, G. Chem. Commun. 2021, 57, 3579.
doi: 10.1039/D1CC00026H |
|
(b) Xu, N.; Zhang, Y.; Chen, W.; Li, P.; Wang, L. Adv. Synth. Catal. 2018, 360, 1199.
doi: 10.1002/adsc.v360.6 |
|
|
(c) Wen, Z. K.; Liu, X. H.; Liu, Y. F.; Chao, J. B. Org. Lett. 2017, 19, 5798.
doi: 10.1021/acs.orglett.7b02753 |
|
| [10] |
(a) Liu, Y.; Zhan, X.; Ji, P.; Xu, J.; Liu, Q.; Luo, W.; Chen, T.; Guo, C. Chem. Commun. 2017, 53, 5346.
doi: 10.1039/C7CC01309D pmid: 26646089 |
|
(b) Shen, T.; Huang, X.; Liang, Y. F.; Jiao, N. Org. Lett. 2015, 17, 6186.
doi: 10.1021/acs.orglett.5b03179 pmid: 26646089 |
|
| [11] |
(a) Chang, M.Y.; Chen, H.Y.; Tsai, Y. L. Org. Lett. 2019, 21, 1832.
doi: 10.1021/acs.orglett.9b00422 |
|
(b) Sun, K.; Zhu, Z.; Sun, J.; Liu, L.; Wang, X. J. Org. Chem. 2016, 81, 1476.
doi: 10.1021/acs.joc.5b02593 |
|
|
(c) Jia, T.; Bellomo, A.; Baina, K. E. L.; Dreher, S. D.; Walsh, P. J. J. Am. Chem. Soc. 2013, 135, 3740.
doi: 10.1021/ja4009776 |
|
| [12] |
(a) Gupta, A.; Rahaman, A.; Bhadra, S. Org. Lett. 2019, 21, 6164.
doi: 10.1021/acs.orglett.9b02424 |
|
(b) Wu, Y.; Huang, Z.; Luo, Y.; Liu, D.; Deng, Y.; Yi, H.; Lee, J. F.; Pao, C. W.; Chen, J. L.; Lei, A. Org. Lett. 2017, 19, 2330.
doi: 10.1021/acs.orglett.7b00865 |
|
| [13] |
(a) Zhu, J.; Guo, Y.; Zhang, Y.; Li, W.; Zhang, P.; Xu, J. Green Chem. 2023, 25, 986.
doi: 10.1039/D2GC04521D |
|
(b) Xu, J.; Liang, C.; Shen, J.; Chen, Q.; Li, W.; Zhang, P. Green Chem. 2023, 25, 1975.
doi: 10.1039/D2GC04909K |
|
|
(c) Xu, J.; Huang, L.; He, L.; Liang, C.; Ouyang, Y.; Shen, J.; Jiang, M.; Li, W. Green Chem. 2021, 23, 6632.
doi: 10.1039/D1GC01899J |
|
|
(d) Xu, J.; He, L.; Liang, C.; Yue, X.; Ouyang, Y.; Zhang, P. ACS Sustainable Chem. Eng. 2021, 9, 13663.
doi: 10.1021/acssuschemeng.1c05237 |
|
| [14] |
(a) Lu, M.; Qin, H.; Lin, Z.; Huang, M.; Weng, W.; Cai, S. Org. Lett. 2018, 20, 7611.
doi: 10.1021/acs.orglett.8b03340 |
|
(b) Budén, M. E.; Bardagí, J. I.; Puiatti, M.; Rossi, R. A. J. Org. Chem. 2017, 82, 8325.
doi: 10.1021/acs.joc.7b00822 |
|
| [15] |
(a) Singsardar, M.; Laru, S.; Mondal, S.; Hajra, A. J. Org. Chem. 2019, 84, 4543.
doi: 10.1021/acs.joc.9b00318 pmid: 30875224 |
|
(b) Zhang, R.; Jin, S.; Liu, Q.; Lin, S.; Yan, Z. J. Org. Chem. 2018, 83, 13030.
doi: 10.1021/acs.joc.8b01508 pmid: 30875224 |
|
|
(c) Ji, X.; Li, D.; Zhou, X.; Huang, H.; Deng, G. J. Green Chem. 2017, 19, 619.
doi: 10.1039/C6GC02271E pmid: 30875224 |
|
|
(d) Xu, Z.; Hang, Z.; Chai, L.; Liu, Z. Q. Org. Lett. 2016, 18, 4662.
doi: 10.1021/acs.orglett.6b02274 pmid: 30875224 |
|
|
(e) Wu, X.; Zhao, Y.; Ge, H. Chem. Sci. 2015, 6, 5978.
doi: 10.1039/C5SC02143J pmid: 30875224 |
|
|
(f) Liu, C.; Zhang, H.; Shi, W.; Lei, A. Chem. Rev. 2011, 111,1780.
doi: 10.1021/cr100379j pmid: 30875224 |
|
|
(g) Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215.
doi: 10.1021/cr100280d pmid: 30875224 |
|
|
(h) Li, C. J. Acc. Chem. Res. 2009, 42, 335.
doi: 10.1021/ar800164n pmid: 30875224 |
|
| [16] |
(a) Tong, H.; Chen, C.; Liu, W.; Pan, Y.; Duan, L. Asian J. Org. Chem. 2019, 8, 479.
doi: 10.1002/ajoc.v8.4 |
|
(b) Liu, W.; Tan, H.; Chen, C.; Pan, Y. Adv. Synth. Catal. 2017, 359, 1594.
doi: 10.1002/adsc.v359.9 |
|
|
(c) Chen, C.; Liu, W.; Zhou, P.; Liu, H. RSC Adv. 2017, 7, 20394.
doi: 10.1039/C7RA02298K |
|
| [17] |
(a) Tan, L.; Chen, C.; Liu, W. Beilstein J. Org. Chem. 2017, 13, 1079.
doi: 10.3762/bjoc.13.107 |
|
(b) Xu, K.; Fang, Y.; Yan, Z.; Zha, Z.; Wang, Z. Org. Lett. 2013, 15, 2148.
doi: 10.1021/ol4006344 |
|
| [18] |
(a) Liu, Y.; Wang, Q. L.; Chen, Z.; Zhou, Q.; Li, H.; Xu, W. Y.; Xiong, B. Q.; Tang, K. W. J. Org. Chem. 2019, 84, 5413.
doi: 10.1021/acs.joc.9b00407 pmid: 30908920 |
|
(b) Li, C.; Jin, T.; Zhang, X.; Li, C.; Jia, X.; Li, J. Org. Lett. 2016, 18, 1916.
doi: 10.1021/acs.orglett.6b00749 pmid: 30908920 |
|
| [19] |
Zhao, Y.; Truhlar, D. G. Theor. Chem. Acc. 2008, 119, 525.
doi: 10.1007/s00214-007-0401-8 |
| [20] |
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A.; Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, revision A.02, Gaussian, Inc., Wallingford, CT, 2009.
|
| [1] | 付雅彤, 孙超凡, 张丹, 金成国, 陆居有. 巢式-碳硼烷硼氢键官能化反应研究进展[J]. 有机化学, 2024, 44(2): 438-447. |
| [2] | 张剑, 梁万洁, 杨艺, 闫法超, 刘会. 联烯胺化合物的区域选择性双官能团化[J]. 有机化学, 2024, 44(2): 335-348. |
| [3] | 李路瑶, 贺忠文, 张振国, 贾振华, 罗德平. 三芳基碳正离子在有机合成中的应用[J]. 有机化学, 2024, 44(2): 421-437. |
| [4] | 范威. O2促进下五元环烯胺的C—H亚胺化[J]. 有机化学, 2023, 43(7): 2492-2498. |
| [5] | 户晓兢, 郭斐翔, 朱润青, 周柄棋, 张涛, 房立真. 对烷氧基酚的合成及其去芳构化后的合成应用[J]. 有机化学, 2023, 43(6): 2239-2244. |
| [6] | 纪健, 刘进华, 管丛, 陈绪文, 赵芸, 刘顺英. 原位生成的磺酸催化N-磺酰基-1,2,3-三氮唑与醇偶联高区域选择性合成N2-取代1,2,3-三氮唑[J]. 有机化学, 2023, 43(3): 1168-1176. |
| [7] | 沈梦涵, 李来强, 周泉, 王洁慧, 王磊. 可见光诱导下喹喔啉酮与吡咯衍生物的氧化偶联[J]. 有机化学, 2023, 43(2): 697-704. |
| [8] | 马彪, 章淼淼, 李占宇, 彭进松, 陈春霞. 无过渡金属催化的Suzuki-Type交叉偶联反应研究进展[J]. 有机化学, 2023, 43(2): 455-470. |
| [9] | 孙婧, 张萌萌, 锅小龙, 王琪, 王陆瑶. 无过渡金属条件下二芳基硒化合物的合成[J]. 有机化学, 2023, 43(12): 4251-4260. |
| [10] | 周鹏, 朱伟明, 张建涛, 肖朵朵, 郭祥峰, 刘卫兵. 钴催化芳基烯烃氧烷基化反应: 快速获得α-烷基取代苯乙酮衍生物[J]. 有机化学, 2023, 43(11): 3939-3944. |
| [11] | 彭菊, 何晓倩, 廖黎丽, 白若鹏, 蓝宇. 取代基电性效应对碳硅还原消除区域选择性调控的理论研究[J]. 有机化学, 2023, 43(10): 3608-3613. |
| [12] | 徐琳琳, 兰美君, 张慕雨, 张永琪, 冯宇豪, 荣良策, 张金鹏. 芳基乙烯β-H区域选择性三氟甲基磺酰化反应[J]. 有机化学, 2022, 42(7): 2134-2139. |
| [13] | 李芳绍, 肖晶, 吴小芳, 王晓熠, 邓金凤, 唐子龙. 无金属条件下酰胺和酯参与的2-取代苯并噁唑衍生物的合成[J]. 有机化学, 2022, 42(6): 1778-1785. |
| [14] | 张智鑫, 翟彤仪, 朱伯汉, 钱鹏程, 叶龙武. 无金属催化炔酰胺分子内[4+2]环化反应合成四氢吲哚衍生物[J]. 有机化学, 2022, 42(5): 1501-1508. |
| [15] | 王馨瑶, 张晴晴, 刘书扬, 李敏, 李海芳, 段春迎, 金云鹤. 可见光诱导无金属条件下交叉脱氢偶联反应合成醌类苄基化衍生物[J]. 有机化学, 2022, 42(5): 1443-1452. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||