有机化学 ›› 2022, Vol. 42 ›› Issue (6): 1747-1758.DOI: 10.6023/cjoc202112040 上一篇 下一篇
研究论文
张同利a, 晏君b, 何敬立a, 寇学振b, 申杰峰a, 刘德龙a,*( ), 张万斌a,b,*(
), 张万斌a,b,*( )
)
收稿日期:2021-12-29
修回日期:2022-02-21
发布日期:2022-03-03
通讯作者:
刘德龙, 张万斌
基金资助:
Tongli Zhanga, Jun Yanb, Jingli Hea, Xuezhen Koub, Jiefeng Shena, Delong Liua( ), Wanbin Zhanga,b(
), Wanbin Zhanga,b( )
)
Received:2021-12-29
Revised:2022-02-21
Published:2022-03-03
Contact:
Delong Liu, Wanbin Zhang
Supported by:文章分享

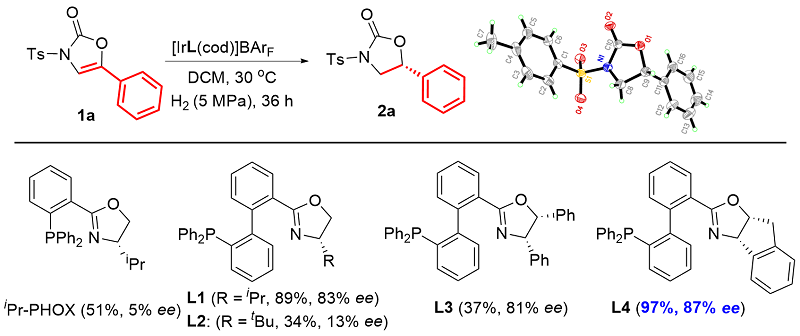
利用轴手性Ir-BiphPHOX催化剂, 首次实现了5-芳基噁唑-2-酮的不对称催化氢化反应, 高收率、高选择性地合成了一系列手性5-芳基噁唑烷-2-酮类化合物. 该反应对多种官能团具有良好的相容性, 所得产物经简单转化可得到多种手性氨基醇类化合物或可衍生出具有生物活性的手性杂环化合物.
张同利, 晏君, 何敬立, 寇学振, 申杰峰, 刘德龙, 张万斌. Ir-BiphPHOX催化的不对称氢化反应合成手性5-芳基噁唑烷-2-酮[J]. 有机化学, 2022, 42(6): 1747-1758.
Tongli Zhang, Jun Yan, Jingli He, Xuezhen Kou, Jiefeng Shen, Delong Liu, Wanbin Zhang. Synthesis of Chiral 5-Aryl-2-oxazolidinones via an Ir-BiphPHOX Catalyzed Enantioselective Hydrogenation[J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1747-1758.
| Entry | Solvent | p(H2)/MPa | Temp./℃ | Conv.b/% | eec/% |
|---|---|---|---|---|---|
| 1 | DCM | 5 | 30 | 97 | 87 |
| 2 | DCE | 5 | 30 | >99 | 93 |
| 3 | PhCl | 5 | 30 | 58 | 93 |
| 4 | Toluene | 5 | 30 | 52 | 69 |
| 5 | o-Xylene | 5 | 30 | 34 | 85 |
| 6 | THF | 5 | 30 | 0 | — |
| 7 | EtOAc | 5 | 30 | 5 | 84 |
| 8 | MeOH | 5 | 30 | 0 | — |
| 9 | DCE | 4 | 30 | 93 | 91 |
| 10 | DCE | 6 | 30 | >99 | 91 |
| 11 | DCE | 5 | 20 | 96 | 90 |
| 12 | DCE | 5 | 50 | >99 | 90 |
| 13d | DCE | 5 | 30 | 82 | 93 |
| Entry | Solvent | p(H2)/MPa | Temp./℃ | Conv.b/% | eec/% |
|---|---|---|---|---|---|
| 1 | DCM | 5 | 30 | 97 | 87 |
| 2 | DCE | 5 | 30 | >99 | 93 |
| 3 | PhCl | 5 | 30 | 58 | 93 |
| 4 | Toluene | 5 | 30 | 52 | 69 |
| 5 | o-Xylene | 5 | 30 | 34 | 85 |
| 6 | THF | 5 | 30 | 0 | — |
| 7 | EtOAc | 5 | 30 | 5 | 84 |
| 8 | MeOH | 5 | 30 | 0 | — |
| 9 | DCE | 4 | 30 | 93 | 91 |
| 10 | DCE | 6 | 30 | >99 | 91 |
| 11 | DCE | 5 | 20 | 96 | 90 |
| 12 | DCE | 5 | 50 | >99 | 90 |
| 13d | DCE | 5 | 30 | 82 | 93 |
| [1] |
(a) Barbachyn, M. R.; Ford, W. Angew. Chem., Int. Ed. 2003, 42, 2010.
doi: 10.1002/anie.200200528 pmid: 15700955 |
|
(b) Mukhtar, T. A.; Wright, G. D. Chem. Rev. 2005, 105, 529.
pmid: 15700955 |
|
|
(c) Wan, Y.-D.; Chen, Z.-X.; Yang, G.-C. Chin. J. Org. Chem. 2005, 25, 1039. (in Chinese)
pmid: 15700955 |
|
|
( 万亚东, 陈祖兴, 杨桂春, 有机化学, 2005, 25, 1039.)
pmid: 15700955 |
|
|
(d) Rensol, A. R.; Luehr, G. W.; Gordeev, M. F. Bioorg. Med. Chem. 2006, 14, 4227.
doi: 10.1016/j.bmc.2006.01.068 pmid: 15700955 |
|
|
(e) Zappia, G.; Menendez, P.; Misiti, D.; Nevola, L.; Botta, B. Mini-Rev. Med. Chem. 2007, 7, 389.
doi: 10.2174/138955707780363783 pmid: 15700955 |
|
|
(f) Das, B.; Rudra, S.; Yadav, A.; Ray, A.; Pandya, M.; Rattan, A.; Mehta, A. Bioorg. Med. Chem. Lett. 2009, 19, 6424.
doi: 10.1016/j.bmcl.2009.09.054 pmid: 15700955 |
|
|
(g) Liang, F.; Chen, L.; Xing, G. Chin. J. Org. Chem. 2009, 29, 1317. (in Chinese)
pmid: 15700955 |
|
|
( 梁芬芬, 陈力, 邢国文, 有机化学, 2009, 29, 1317.)
pmid: 15700955 |
|
|
(h) Shaw, K. J.; Barbachyn, M. R. Ann. N. Y. Acad. Sci. 2011, 1241, 48.
pmid: 15700955 |
|
|
(i) Zhang, H.-Z.; Zhou, C.-H.; Geng, R.-X.; Ji, Q.-G. Chin. J. Org. Chem. 2011, 31, 1963. (in Chinese)
pmid: 15700955 |
|
|
( 张慧珍, 周成合, 耿蓉霞, 吉庆刚, 有机化学, 2011, 31, 1963.)
pmid: 15700955 |
|
| [2] |
Fujimoto, J.; Okamoto, R.; Noguchi, N. J. Med. Chem. 2017, 60, 8963.
doi: 10.1021/acs.jmedchem.7b01210 pmid: 29023121 |
| [3] |
Slassi, A.; Joseph, B.; Ma, F.; Egle, I.; Clayton, I.; Isaac, M.; Swierczek, K. US 20070275966, 2007.
|
| [4] |
(a) Onodera, G.; Watabe, K.; Matsubara, M.; Oda, K.; Kezuka, S.; Takeuchia, R. Adv. Synth. Catal. 2008, 350, 2725.
doi: 10.1002/adsc.200800333 |
|
(b) Li, W.; Wollenburg, M.; Glorius, F. Chem. Sci. 2018, 9, 6260.
doi: 10.1039/C8SC01869C |
|
| [5] |
Han, X.; Liu, R.; Ji, L. J. Chromatogr. B 2016, 1008, 108.
doi: 10.1016/j.jchromb.2015.11.017 |
| [6] |
Lu, C.; Luo, Z.; Huang, L. Tetrahedron: Asymmetry 2011, 22, 722.
|
| [7] |
Wei, S.; Messerer, R.; Tsogoeva, S. B. Chem.-Eur. J. 2011, 17, 14380.
doi: 10.1002/chem.201102931 |
| [8] |
For selected examples: (a) Puschin, N. A.; Mitic, R. V. Leibigs Ann. Chem. 1937, 532, 300.
pmid: 10814394 |
|
(b) Newma, M. S.; Kutner, A. J. Am. Chem. Soc. 1951, 73, 4199.
doi: 10.1021/ja01153a047 pmid: 10814394 |
|
|
(c) Ben, I. D. J. Am. Chem. Soc. 1956, 78, 4962.
doi: 10.1021/ja01600a042 pmid: 10814394 |
|
|
(d) Dyen, M. E.; Swern, D. Chem. Rev. 1967, 67, 197.
pmid: 10814394 |
|
|
(e) Yoshida, T.; Kambe, N.; Murai, S.; Sonoda, N. Tetrahedron Lett. 1986, 27, 3037.
doi: 10.1016/S0040-4039(00)84710-3 pmid: 10814394 |
|
|
(f) Pridgen, L. N.; Prol, J.; Alexander, B. J. Org. Chem. 1989, 54, 3231.
doi: 10.1021/jo00274a058 pmid: 10814394 |
|
|
(g) Ager, D. J.; Prakash, I.; Schaad, D. R. Chem. Rev. 1996, 96, 835.
doi: 10.1021/cr9500038 pmid: 10814394 |
|
|
(h) Kudo, N.; Taniguchi, M.; Furuta, S.; Sato, K.; Endo, T.; Honma, T. J. Agric. Food Chem. 1998, 46, 5305.
doi: 10.1021/jf980281i pmid: 10814394 |
|
|
(i) Cynamon, M. H.; Klemens, S. P.; Sharpe, C. A.; Chase, S. Antimicrob. Agents Chemother. 1999, 43, 1189.
pmid: 10814394 |
|
|
(j) Wang, G.; Hollingsworth, R. I. Tetrahedron: Asymmetry 2000, 11, 4429.
pmid: 10814394 |
|
|
(k) Gabriele, B.; Salerno, G.; Costa, M.; Chiusoli, G. P. Org. Lett. 2000, 2, 625.
pmid: 10814394 |
|
|
(l) Ariza, X.; Pineda, O.; Urpi, F.; Vilarrasa, J. Tetrahedron Lett. 2001, 42, 4995.
doi: 10.1016/S0040-4039(01)00901-7 pmid: 10814394 |
|
|
(m) Liu, J. M.; Peng, X. G.; Liu, J. H.; Zheng, S. Z.; Sun, W.; Xia, C. G. Tetrahedron Lett. 2007, 48, 929.
doi: 10.1016/j.tetlet.2006.12.028 pmid: 10814394 |
|
|
(n) Patil, Y. P.; Tambade, P. J.; Jagtap, S. R.; Bhanage, B. M. J. Mol. Catal. A: Chem. 2008, 289, 14.
doi: 10.1016/j.molcata.2008.03.019 pmid: 10814394 |
|
| [9] |
(a) Iwama, S.; Katsumura, S. Bull. Chem. Soc. Jpn. 1994, 67, 3363.
doi: 10.1246/bcsj.67.3363 pmid: 11667610 |
|
(b) Tingoli, M.; Testaferri, L.; Temparini, A.; Tiecco, M. J. Org. Chem. 1996, 61, 7085.
pmid: 11667610 |
|
|
(c) Ueno, A.; Kayaki, Y.; Ikariya, T. Green Chem. 2013, 15, 425.
doi: 10.1039/C2GC36414J pmid: 11667610 |
|
|
(d) Khan, A.; Xing, J.; Zhao, J.; Kan, Y.; Zhang, W.; Zhang, Y. J. Chem.-Eur. J. 2015, 21, 120.
doi: 10.1002/chem.201405830 pmid: 11667610 |
|
| [10] |
(a) Lebal, H.; Huard, K.; Lectard, S. J. Am. Chem. Soc. 2005, 127, 14198.
doi: 10.1021/ja0552850 |
|
(b) Barman, D. N.; Nicholas, K. M. Eur. J. Org. Chem. 2011, 2011, 908.
doi: 10.1002/ejoc.201001160 |
|
| [11] |
Wan, N. W.; Tian, J. W.; Zhou, X. Y.; Wang, H. H.; Cui, B. D.; Han, W. Y.; Chen, Y. Z. Adv. Synth. Catal. 2019, 361, 4651.
doi: 10.1002/adsc.201900786 |
| [12] |
(a) Noyori, R.; Takaya, H. Acc. Chem. Res. 1990, 23, 345.
doi: 10.1021/ar00178a005 pmid: 24568181 |
|
(b) Tang, W.; Zhang, X. Chem. Rev. 2003, 103, 3029.
doi: 10.1021/cr020049i pmid: 24568181 |
|
|
(c) Johnson, N. B.; Lennon, I. C.; Moran, P. H.; Raasden, J. Acc. Chem. Res. 2007, 40, 1291.
doi: 10.1021/ar700114k pmid: 24568181 |
|
|
(d) Ma, Y.-H.; Zhang, Y.-J.; Zhang, W.-B. Chin. J. Org. Chem. 2007, 27, 289. (in Chinese)
doi: 10.1002/cjoc.200990046 pmid: 24568181 |
|
|
( 马元辉, 张勇健, 张万斌, 有机化学, 2007, 27, 289.)
pmid: 24568181 |
|
|
(e) Li, Y.; Zheng, Y.; Tian, F.; Zhang, Y.; Zhang, W. Chin. J. Org. Chem. 2009, 29, 1487. (in Chinese)
pmid: 24568181 |
|
|
( 李亚玺, 郑玉林, 田丰涛, 张勇健, 张万斌, 有机化学, 2009, 29, 1487.)
pmid: 24568181 |
|
|
(f) Xie, J. H.; Zhu, S. F.; Zhou, Q. L. Chem. Rev. 2011, 111, 1713.
doi: 10.1021/cr100218m pmid: 24568181 |
|
|
(g) Verendel, J. J.; Pamies, O.; Dieguez, M.; Andersson, P. G. Chem. Rev. 2014, 114, 2130.
doi: 10.1021/cr400037u pmid: 24568181 |
|
|
(h) Xie, J.; Zhou, Q. Acta Chim. Sinica 2014, 72, 778. (in Chinese)
pmid: 24568181 |
|
|
( 谢建华, 周其林, 化学学报, 2014, 72, 778.)
doi: 10.6023/A14050364 pmid: 24568181 |
|
|
(i) Wang, Y.; Zhang, Z.; Zhang, W. Chin. J. Org. Chem. 2015, 35, 528. (in Chinese)
doi: 10.6023/cjoc201502017 pmid: 24568181 |
|
|
( 王英杰, 张振锋, 张万斌, 有机化学, 2015, 35, 528.)
doi: 10.6023/cjoc201502017 pmid: 24568181 |
|
|
(j) Yuan, Q.; Zhang, W. Chin. J. Org. Chem. 2016, 36, 274. (in Chinese)
doi: 10.6023/cjoc201509016 pmid: 24568181 |
|
|
( 袁乾家, 张万斌, 有机化学, 2016, 36, 274.)
doi: 10.6023/cjoc201509016 pmid: 24568181 |
|
|
(k) Wang, Z.; Zhang, Z.; Liu, Y.; Zhang, W. Chin. J. Org. Chem. 2016, 36, 447. (in Chinese)
doi: 10.6023/cjoc201512009 pmid: 24568181 |
|
|
( 王志惠, 张振锋, 刘燕刚, 张万斌, 有机化学, 2016, 36, 447.)
doi: 10.6023/cjoc201512009 pmid: 24568181 |
|
|
(l) Zhang, Z.; Butt, N. A.; Zhang, W. Chem. Rev. 2016, 116, 14769.
doi: 10.1021/acs.chemrev.6b00564 pmid: 24568181 |
|
|
(m) Wiesenfeldt, M. P.; Nairoukh, Z.; Dalton, T.; Glorius, F. Angew. Chem., Int. Ed. 2019, 58, 10460.
doi: 10.1002/anie.201814471 pmid: 24568181 |
|
|
(n) Gu, X.; Li, X.; Xie, J.; Zhou, Q. Acta Chim. Sinica 2019, 77, 598. (in Chinese)
pmid: 24568181 |
|
|
( 顾雪松, 李校根, 谢建华, 周其林, 化学学报, 2019, 77, 598.)
doi: 10.6023/A19050166 pmid: 24568181 |
|
|
(o) Dou, X.; Liu, D.; Zhang, W. Chin. J. Pharm. 2020, 51, 145. (in Chinese)
pmid: 24568181 |
|
|
( 窦骁勇, 刘德龙, 张万斌, 中国医药工业杂志, 2020, 51, 145.)
pmid: 24568181 |
|
|
(p) Chen, J.; Zhang, W. Chin. J. Org. Chem. 2020, 40, 4372. (in Chinese)
doi: 10.6023/cjoc202000086 pmid: 24568181 |
|
|
( 陈建中, 张万斌, 有机化学, 2020, 40, 4372.)
doi: 10.6023/cjoc202000086 pmid: 24568181 |
|
|
(q) Zheng, Y.; Zhang, X.; Zhang, R.; Ma, B. Green Synth. Catal. 2021, 2, 393.
pmid: 24568181 |
|
| [13] |
Wang, Q.; Tan, X.; Zhu, Z.; Dong, X. Q.; Zhang, X. Tetrahedron Lett. 2016, 57, 658.
doi: 10.1016/j.tetlet.2015.12.105 |
| [14] |
Li, W.; Wollenburg, M.; Glorius, F. Chem. Sci. 2018, 9, 6260.
doi: 10.1039/C8SC01869C |
| [15] |
Liu, Y.; Yi, Z.; Yang, X.; Wang, H.; Yin, C.; Wang, M.; Dong, X. Q.; Zhang, X. ACS Catal. 2020, 10, 11153.
doi: 10.1021/acscatal.0c02569 |
| [16] |
(a) Tian, F.; Yao, D.; Zhang, Y. J.; Zhang, W. Tetrahedron 2009, 65, 9609.
doi: 10.1016/j.tet.2009.09.053 pmid: 34213913 |
|
(b) Tian, F.; Yao, D.; Liu, Y.; Xie, F.; Zhang, W. Adv. Synth. Catal. 2010, 352, 1841.
doi: 10.1002/adsc.201000185 pmid: 34213913 |
|
|
(c) Liu, Y.; Yao, D.; Li, K.; Tian, F.; Xie, F.; Zhang, W. Tetrahedron 2011, 67, 8445.
doi: 10.1016/j.tet.2011.09.023 pmid: 34213913 |
|
|
(d) Liu, Y.; Zhang, W. Angew. Chem., Int. Ed. 2013, 52, 2203.
doi: 10.1002/anie.201209126 pmid: 34213913 |
|
|
(e) Liu, Y.; Gridnev, I. D.; Zhang, W. Angew. Chem., Int. Ed. 2014, 53, 1901.
doi: 10.1002/anie.201309677 pmid: 34213913 |
|
|
(f) Xia, J.; Yang, G.; Zhuge, R.; Liu, Y.; Zhang, W. Chem. Eur. J. 2016, 22, 18354.
doi: 10.1002/chem.201604298 pmid: 34213913 |
|
|
(g) Quan, M.; Tang, L.; Shen, J.; Yang, G.; Zhang, W. Chem. Commun. 2017, 53, 609.
doi: 10.1039/C6CC08759K pmid: 34213913 |
|
|
(h) Xia, J.; Nie, Y.; Yang, G.; Liu, Y.; Zhang, W. Org. Lett. 2017, 19, 4884.
doi: 10.1021/acs.orglett.7b02341 pmid: 34213913 |
|
|
(i) Meng, K.; Xia, J.; Wang, Y.; Zhang, X.; Yang, G.; Zhang, W. Org. Chem. Front. 2017, 4, 1601.
doi: 10.1039/C7QO00248C pmid: 34213913 |
|
|
(j) Xia, J.; Nie, Y.; Yang, G.; Liu, Y.; Gridnev, I. D.; Zhang, W. Chin. J. Chem. 2018, 36, 612.
doi: 10.1002/cjoc.201800088 pmid: 34213913 |
|
|
(k) Wang, Y.; Xia, J.; Yang, G.; Zhang, W. Tetrahedron 2018, 74, 477.
doi: 10.1016/j.tet.2017.12.015 pmid: 34213913 |
|
|
(l) Yan, J.; Nie, Y.; Gao, F.; Yuan, Q.; Xie, F.; Zhang, W. Tetrahedron 2021, 84, 132003.
doi: 10.1016/j.tet.2021.132003 pmid: 34213913 |
|
|
(m) Nie, Y.; Li, J.; Yan, J.; Yuan, Q.; Zhang, W. Org. Lett. 2021, 23, 5373.
doi: 10.1021/acs.orglett.1c01701 pmid: 34213913 |
|
| [17] |
Tobias, A.; Goran, H. Org. Lett. 2009, 11, 503.
doi: 10.1021/ol802243d pmid: 19123840 |
| [1] | 陈宛婷, 钟雄威, 邢佳乐, 吴昌书, 高杨. C—N轴手性化合物的不对称催化合成研究进展[J]. 有机化学, 2024, 44(2): 349-377. |
| [2] | 姜权彬. 经由氮杂邻联烯醌中间体合成轴手性化合物的研究进展[J]. 有机化学, 2024, 44(1): 159-172. |
| [3] | 于士航, 刘嘉威, 安碧玉, 边庆花, 王敏, 钟江春. 黑腹尼虎天牛接触性信息素的不对称合成[J]. 有机化学, 2024, 44(1): 301-308. |
| [4] | 程春霞, 吴露平, 沙风, 伍新燕. 手性叔膦-酰胺不对称催化香豆素与Morita-Baylis-Hillman碳酸酯之间的插烯烯丙基烷基化反应[J]. 有机化学, 2023, 43(9): 3188-3195. |
| [5] | 王文芳. 过渡金属催化不对称C—H硼化反应研究进展[J]. 有机化学, 2023, 43(9): 3146-3166. |
| [6] | 罗诚, 尹艳丽, 江智勇. P-手性膦氧化物的不对称合成研究进展[J]. 有机化学, 2023, 43(6): 1963-1976. |
| [7] | 褚杨杨, 韩召斌, 丁奎岭. 动力学拆分在过渡金属催化的不对称(转移)氢化中的应用研究[J]. 有机化学, 2023, 43(6): 1934-1951. |
| [8] | 刘洋, 黄翔, 王敏, 廖建. 铜催化环酮亚胺与β,γ-不饱和N-酰基吡唑不对称Mannich-Type反应[J]. 有机化学, 2023, 43(4): 1499-1509. |
| [9] | 杨晓东, 郑小康, 董海亮, 孙静, 王华. 圆偏振热活化延迟荧光材料及其器件的研究进展[J]. 有机化学, 2023, 43(4): 1292-1309. |
| [10] | 曹伟地, 刘小华. 不对称催化质子化构建α-叔碳羰基化合物研究进展[J]. 有机化学, 2023, 43(3): 961-973. |
| [11] | 向勋, 何照林, 董秀琴. 钯和手性磷酸协同催化高效构建手性分子的研究进展[J]. 有机化学, 2023, 43(3): 791-808. |
| [12] | 陈宇亮, 贺凤开, 王思云, 贾鼎成, 刘亚群, 黄毅勇. 手性磷酸催化α-全碳季碳醛的不对称烯丙基化动力学拆分[J]. 有机化学, 2023, 43(12): 4294-4302. |
| [13] | 吴利城, 伍贤青, 曲景平, 陈宜峰. Quinim配体的探索及其在镍催化烯烃的不对称胺甲酰基-烷基化反应的应用[J]. 有机化学, 2023, 43(12): 4239-4250. |
| [14] | 王倩, 刘雨奇, 吴宗铨. 手性螺旋聚合物的合成和结构控制[J]. 有机化学, 2023, 43(12): 4141-4146. |
| [15] | 刘甜甜, 段新红. 不对称傅-克反应在构建手性3-取代吲哚中的研究进展[J]. 有机化学, 2023, 43(11): 3695-3712. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||