有机化学 ›› 2022, Vol. 42 ›› Issue (6): 1759-1769.DOI: 10.6023/cjoc202111015 上一篇 下一篇
研究论文
袁飞, 赵艳, 郭青松, 尹福丹, 赖金荣, 念倍芳, 张明, 汤峨*( )
)
收稿日期:2021-11-07
修回日期:2022-02-07
发布日期:2022-02-25
通讯作者:
汤峨
作者简介:基金资助:
Fei Yuan, Yan Zhao, Qingsong Guo, Fudan Yin, Jinrong Lai, Beifang Nian, Ming Zhang, E Tang( )
)
Received:2021-11-07
Revised:2022-02-07
Published:2022-02-25
Contact:
E Tang
About author:Supported by:文章分享
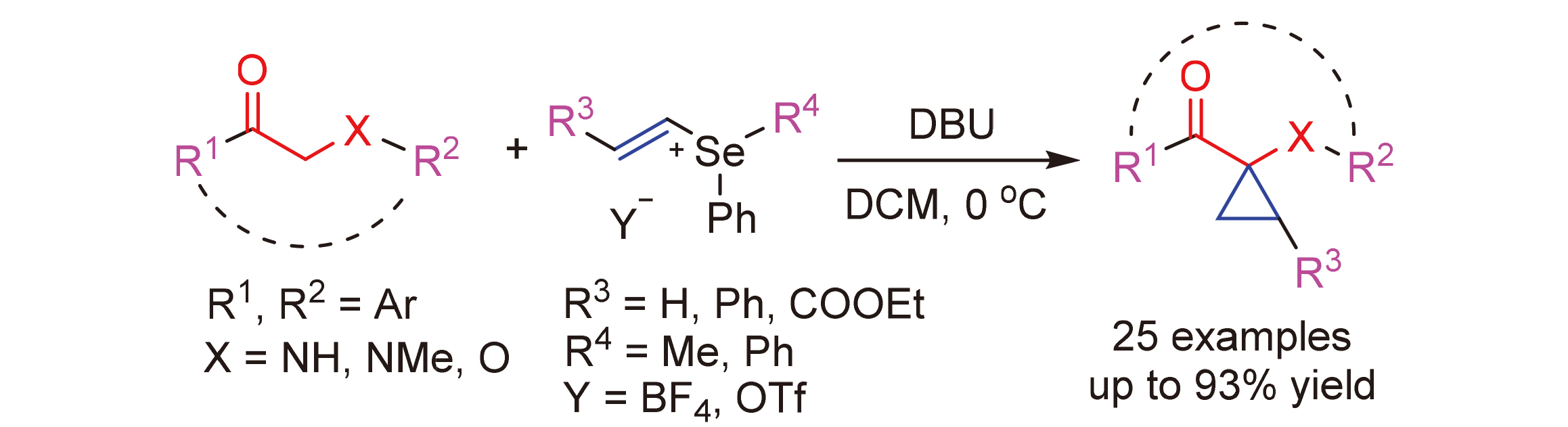
发展了一种“一锅法”高效合成1-[1-(胺基)环丙基]酮化合物的新方法. 在碱的作用下, β-胺基酮化合物与甲基苯基乙烯基硒四氟硼酸盐发生亲核加成/质子转移/亲核取代脱硒醚的串联反应, 以70%~93%的产率得到1-[1-(胺基)环丙基]酮化合物. 该方法具有条件温和、操作简单、官能团兼容性良好、区域选择性好等优点. 用溴素和肼对副产物甲基苯基硒醚与乙烯基硒盐进行简单处理, 就可以制备出合成硒盐的通用原料二苯基二硒醚, 继而实现乙烯基硒盐的再生和重复利用, 大大地提高了高价硒试剂的利用效率和该方法的工业应用价值.
袁飞, 赵艳, 郭青松, 尹福丹, 赖金荣, 念倍芳, 张明, 汤峨. 乙烯基硒盐参与的串联反应合成1-[1-(胺基)环丙基]酮化合物[J]. 有机化学, 2022, 42(6): 1759-1769.
Fei Yuan, Yan Zhao, Qingsong Guo, Fudan Yin, Jinrong Lai, Beifang Nian, Ming Zhang, E Tang. Synthesis of 1-[1-(Amino)cyclopropyl]ketones by Tandem Reaction Involving Vinyl Selenium Salt[J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1759-1769.


| Entry | Solvent | Base | T/℃ | Time/min | Yield b/% of 3a |
|---|---|---|---|---|---|
| 1 | DCM | DBU | 0 | 60 | 89 |
| 2 | DCM | NaH | 0 | 60 | 20 |
| 3 | DCM | TEA | 0 | 60 | 0 |
| 4 | DCM | K2CO3 | 0 | 60 | 0 |
| 5 | DCM | Cs2CO3 | 0 | 60 | 0 |
| 6 | THF | DBU | 0 | 60 | 80 |
| 7 | CH3CN | DBU | 0 | 60 | 62 |
| 8 | Toluene | DBU | 0 | 60 | 48 |
| 9 | DCM | DBU | 25 | 60 | 87 |
| 10 | DCM | DBU | 0 | 80 | 89 |
| 11 | DCM | DBU | 0 | 40 | 73 |
| 12 | DCM | DBU | 0 | 20 | 40 |
| 13c | DCM | DBU | 0 | 60 | 42 |
| Entry | Solvent | Base | T/℃ | Time/min | Yield b/% of 3a |
|---|---|---|---|---|---|
| 1 | DCM | DBU | 0 | 60 | 89 |
| 2 | DCM | NaH | 0 | 60 | 20 |
| 3 | DCM | TEA | 0 | 60 | 0 |
| 4 | DCM | K2CO3 | 0 | 60 | 0 |
| 5 | DCM | Cs2CO3 | 0 | 60 | 0 |
| 6 | THF | DBU | 0 | 60 | 80 |
| 7 | CH3CN | DBU | 0 | 60 | 62 |
| 8 | Toluene | DBU | 0 | 60 | 48 |
| 9 | DCM | DBU | 25 | 60 | 87 |
| 10 | DCM | DBU | 0 | 80 | 89 |
| 11 | DCM | DBU | 0 | 40 | 73 |
| 12 | DCM | DBU | 0 | 20 | 40 |
| 13c | DCM | DBU | 0 | 60 | 42 |
| [1] |
(a) Liu, H.-W.; Walsh, C. T. The Chemistry of the Cyclopropyl Group, Ed.: Rappoport, Z., John Wiley & Sons Ltd., New York, 1987.
pmid: 12683792 |
|
(b) Wessjohann, L. A.; Brandt, W. Chem. Rev. 2003, 103, 1625.
pmid: 12683792 |
|
| [2] |
Wan, L.-Z.; Wang, H. Chemistry (Huaxue Tongbao) 2019, 82, 963. (in Chinese)
|
|
( 万灵子, 王晗, 化学通报, 2019, 82, 963.)
|
|
| [3] |
(a) Reissig, H. U.; Zimmer, R. Chem. Rev. 2003, 103, 1151.
|
|
(b) Gnad, F.; Reiser, O. Chem. Rev. 2003, 103, 1603.
doi: 10.1021/cr010015v |
|
|
(c) Cohen, Y.; Cohen, A.; Marek, I. Chem. Rev. 2021, 121, 140.
doi: 10.1021/acs.chemrev.0c00167 |
|
|
(d) Ebner, C.; Carreira, E. M. Chem. Rev. 2017, 117, 11651.
doi: 10.1021/acs.chemrev.6b00798 |
|
|
(e) Pirenne, V.; Muriel, B.; Waser, J. Chem. Rev. 2021, 121, 227.
doi: 10.1021/acs.chemrev.0c00109 |
|
| [4] |
(a) Barone, V.; Fraternali, F.; Cristinziano, P. L.; Lelj, F.; Rosa, A. Biopolymers 1988, 27, 1673.
doi: 10.1002/bip.360271011 pmid: 9151253 |
|
(b) Benedetti, E.; Di Blasio, B.; Pavone, V.; Pedone, C.; Santini, A.; Crisma, M.; Valle, G.; Toniolo, C. Biopolymers 1989, 28, 175.
doi: 10.1002/bip.360280119 pmid: 9151253 |
|
|
(c) Toniolo, C. Int. J. Pept. Protein Res. 1990, 35, 287.
pmid: 9151253 |
|
|
(d) Burgess, K.; Ke, C. Y. J. Pept. Res. 1997, 49, 201.
pmid: 9151253 |
|
| [5] |
(a) Burgess, K.; Li, W.; Lim, D.; Moye-Sherman, D. Biopolymers 1997, 42, 439.
pmid: 11782172 |
|
(b) Martin, S. F.; Dwyer, M. P.; Hartmann, B.; Knight, K. S. J. Org. Chem. 2000, 65, 1305
pmid: 11782172 |
|
|
(c) Davidson, J. P.; Lubman, O.; Rose, T.; Waksman, G.; Martin, S. F. J. Am. Chem. Soc. 2002, 124, 205.
pmid: 11782172 |
|
| [6] |
(a) Ner, S. K.; Suckling, C. J.; Bell, A. R.; Wrigglesworth, R. Chem. Commun. 1987, 480.
|
|
(b) Kemp, A.; Tedford, C.; Suckling, C. J. Bioorg. Med. Chem. Lett. 1991, 1, 557.
doi: 10.1016/S0960-894X(01)80465-0 |
|
|
(c) Hilier, M. C.; Davidson, J. P.; Martin, S. F. J. Org. Chem. 2001, 66, 1657.
doi: 10.1021/jo001257i |
|
| [7] |
Andrew, M.; Dominique, G. Nat. Prod. Res. 2005, 19, 639.
pmid: 16076632 |
| [8] |
Talele, T. T. J. Med. Chem. 2016, 59, 8712.
doi: 10.1021/acs.jmedchem.6b00472 |
| [9] |
(a) Han, W.; Hu, Z.; Jiang, X.; Wasserman, Z. R.; Decicco, C. P. Bioorg. Med. Chem. Lett. 2003, 13, 1111.
|
|
(b) Lamarre, D.; Anderson, P. C.; Bailey, M.; Beaulieu, P.; Bolger, G.; Bonneau, P.; Bos, M.; Cameron, D. R.; Cariter, M.; Cordingley, M. G.; Faucher, A.-M.; Goudreau, N.; Kawai, S. H.; Kukolj, G.; Lagace, L.; Laplante, S. R.; Narjes, H.; Poupart, M.-A.; Rancourt, J.; Sentjens, R. E.; St George, R.; Simoneau, B.; Steinmann, G.; Thiebeault, D.; Tsantrizos, Y. S.; Weldon, S. M.; Yong, C.-L.; Llinas-Brunet, M. Nature 2003, 426, 186.
doi: 10.1038/nature02099 |
|
| [10] |
Kawada, M.; Kawano, Y.; Sugihara, H.; Takei, S.; Imada, I. Chem. Pharm. Bull. 1981, 29, 1900.
doi: 10.1248/cpb.29.1900 |
| [11] |
Brotherton, C. A.; Wilson, M.; Byrd, G.; Balskus, E. P. Org. Lett. 2015, 17, 1545.
doi: 10.1021/acs.orglett.5b00432 pmid: 25753745 |
| [12] |
Serusi, L.; Soddu, F.; Cuccu, F.; Peretti, G.; Luridiana, A.; Secci, F.; Caboni, P.; Aitken, D. J.; Frongia, A. Adv. Synth. Catal. 2020, 362, 4159.
doi: 10.1002/adsc.202000541 |
| [13] |
Mao, Z.-J.; Qu, H.-J.; Zhao, Y.-Y.; Lin, X.-F. Chem. Commun. 2012, 48, 9927.
doi: 10.1039/c2cc35235d |
| [14] |
Wirth, T. Organoselenium Chemistry (Synthesis and Reactions), Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2012, pp. 204-211.
|
| [15] |
(a) Tang, E; Wang, W. L.; Zhao, Y. J.; Zhang, M.; Dai, X. Org. Lett. 2016, 18, 176.
doi: 10.1021/acs.orglett.5b03157 pmid: 26882088 |
|
(b) Tang, E; Zhao, Y. J.; Li, W.; Wang, W. L.; Zhang, M.; Dai, X. Org. Lett. 2016, 18, 912.
doi: 10.1021/acs.orglett.5b03579 pmid: 26882088 |
|
|
(c) Tang, E; Zhao, Y. J.; Zhang, M.; Dai, X.; Wang, W. L. Heterocycles 2016, 92, 708.
doi: 10.3987/COM-15-13402 pmid: 26882088 |
|
|
(d) Sun, Q.; Liao, M. H.; Zhao, Y.; Li, Y. X.; Liu, S. S.; Mao, D. S.; Tang, E. Heterocycles 2019, 98, 1563.
doi: 10.3987/COM-19-14162 pmid: 26882088 |
|
|
(e) Liao, M. H.; Zhang, M.; Hu, D. H.; Zhang, R. H.; Zhao, Y.; Liu, S. S.; Li, Y. X.; Xiao, W. L.; Tang, E Org. Biomol. Chem. 2020, 18, 3941.
doi: 10.1039/D0OB90071K pmid: 26882088 |
|
| [16] |
(a) Tang, Y.; Ye, S.; Sun, X.-L. Synlett 2005, 2720.
|
|
(b) Maryanoff, B. E.; Reitz, A. B. Chem. Rev. 1989, 89, 863.
doi: 10.1021/cr00094a007 |
|
|
(c) Boutagy, J.; Thomas, R. Chem. Rev. 1974, 74, 87.
doi: 10.1021/cr60287a005 |
|
|
(d) Aggarwal, V. K.; Winn, C. L. Acc. Chem. Res. 2004, 37, 611.
doi: 10.1021/ar030045f |
|
| [17] |
Watanabe, S.-I.; Kusumoto, T.; Yoshida, C.; Kataoka, T. Chem. Commun. 2001, 839.
|
| [18] |
(a) Borzecka, W.; Lavandera, I.; Gotor, V. J. Org. Chem. 2013, 78, 7312.
doi: 10.1021/jo400962c |
|
(b) Pal, M.; Swamy, N. K.; Hameed, P. S.; Padakanti, S.; Yeleswarapu, K. R. Tetrahedron 2004, 60, 3987.
doi: 10.1016/j.tet.2004.03.036 |
|
| [19] |
Watanabe, Y.; Ueno, Y.; Toru, T. Bull. Chem. Soc. Jpn. 1993, 66, 2042.
doi: 10.1246/bcsj.66.2042 |
| [20] |
Gilbert, B. B.; Eey, S. T.-C.; Ryabchuk, P.; Garry, O.; Denmark, S. E. Tetrahedron 2019, 75, 4086.
|
| [21] |
(a) Mato, M.; Franchino, A.; Garcı́a-Morales, C.; Echavarren, A. M. Chem. Rev. 2021, 121, 8613.
|
|
(b) Gao, J.-S.; Bian, Q.-H.; Guo, H.-C. Chin. J. Org. Chem. 2007, 27, 438. (in Chinese)
|
|
|
( 高金山, 边庆花, 郭洪超, 有机化学, 2007, 27, 438.)
|
|
|
(c) Liang, J.; Ma, H.-F.; Ablajan, K. Chin. J. Org. Chem. 2019, 39, 3169. (in Chinese)
doi: 10.6023/cjoc201904028 |
|
|
( 梁杰, 马会芳, 阿布拉江•克依木, 有机化学, 2019, 39, 3169.)
doi: 10.6023/cjoc201904028 |
|
|
(d) Wang, M.; Chen, J.; Wu, X.-Y.; Deng, H.-M.; Zhang, H.; Shao, M.; Cao, W.-G. Chin. J. Org. Chem. 2009, 29, 1611. (in Chinese)
|
|
|
( 王猛, 陈杰, 吴小余, 邓红梅, 张慧, 邵敏, 曹卫国, 有机化学, 2009, 29, 1611.)
|
|
|
(e) Su, G.-F.; Pan, C.-X.; Mou, H.-T.; Zeng, L.-M.; Yu, K.-B. Acta Chim. Sinica 2004, 62, 1941. (in Chinese)
|
|
|
( 苏桂发, 潘成学, 牟红涛, 曾陇梅, 郁开北, 化学学报, 2004, 62, 1941.)
|
|
|
(f) Shi, Z.-J.; Liu, M.; Liang, C. Chin. J. Synth. Chem. 2008, 16, 546. (in Chinese)
|
|
|
( 施志坚, 刘梅, 梁超, 合成化学, 2008, 16, 546.)
|
|
|
(g) Li, X.-D.; Fan, X.-E.; Zhang, G.-Y.; Chen, Q.-H. Chemistry (Huaxue Tongbao) 2005, 68, 209. (in Chinese)
|
|
|
( 李晓冬, 范雪娥, 张桂英, 陈庆华, 化学通报, 2005, 68, 209.)
|
|
|
(h) Gao, T.-Y.; Shi, Z.-J.; Cao, W.-G.; Deng, H.-M. Chin. J. Synth. Chem. 2009, 17, 566. (in Chinese)
|
|
|
( 高天云, 施志坚, 曹卫国, 姜海燕, 邓红梅, 合成化学, 2009, 17, 566.)
|
|
|
(i) Zhang, X.-L.; Huang, H.-H.; Chen, Q.-H. Chin. J. Org. Chem. 2002, 22, 411. (in Chinese)
doi: 10.1002/cjoc.20040220502 |
|
|
( 张熊禄, 黄海洪, 陈庆华, 有机化学, 2002, 22, 411.)
|
|
|
(j) Jin, W.-B.; Yuan, H.; Tang, G.-L. Chin. J. Org. Chem. 2018, 38, 2324. (in Chinese)
doi: 10.6023/cjoc201805059 |
|
|
( 金文兵, 袁华, 唐功利, 有机化学, 2018, 38, 2324.)
doi: 10.6023/cjoc201805059 |
|
| [22] |
Luan, B.; Catalina, S.; Lorenzo, T.; Marcello, T. Tetrahedron: Asymmetry 2009, 20, 1506.
|
| [23] |
Watanabe, Y.; Ueno, Y.; Toru, T. Bull. Chem. Soc. Jpn. 1993, 66, 2042.
doi: 10.1246/bcsj.66.2042 |
| [24] |
Francesca, G. N.; Bonifacio, M.; Eder, J. L.; Paul, E.; Claudio, S. Molecules 2020, 25, 2018.
doi: 10.3390/molecules25092018 |
| [25] |
Guo, S.-Q.; Zhang, N.-N.; Tang, X.-Z.; Mao, Z.-F.; Zhang, X.-J.; Yan, M.; Xuan, Y.-N. Chin. Chem. Lett. 2019, 30, 406.
doi: 10.1016/j.cclet.2018.08.021 |
| [26] |
Mao, Z.-J.; Qu, H.-J.; Zhao, Y.-Y.; Lin, X.-F. Chem. Commun. 2012, 48, 9927.
doi: 10.1039/c2cc35235d |
| [1] | 曾成富, 何媛, 李清, 董琳. Ir(III)催化新型三组分串联三氟乙氧基化反应并一锅法构建复杂酰胺化合物[J]. 有机化学, 2023, 43(3): 1115-1123. |
| [2] | 王永玲, 张铁欣, 张栩铭, 孙晗扬, 冷津瑶, 李亚明. 可见光催化N-芳基乙醛酸亚胺脱羧烷基化合成非天然氨基酸衍生物[J]. 有机化学, 2023, 43(12): 4284-4293. |
| [3] | 李硕, 王明亮, 周来运, 王兰芝. 磁性纳米负载对甲苯磺酸催化串联合成稠合多环的1,5-苯并氧氮杂䓬类化合物[J]. 有机化学, 2023, 43(11): 3977-3988. |
| [4] | 景智霞, 杜建喜, 蒋平, 阿布拉江•克依木. 四丁基碘化胺介导烷基酰胺与酰肼一锅法构建1,3,4-噁二唑衍生物[J]. 有机化学, 2023, 43(11): 3930-3938. |
| [5] | 乃比江•赛米, 张蕾, 买地娜•沙拉木, 曾竟, 阿布都热西提•阿布力克木. 硫代磺酸酯和磺酰卤的绿色合成研究[J]. 有机化学, 2023, 43(1): 236-243. |
| [6] | 石云, 肖婷, 夏冬, 杨文超. 三氟甲硫基自由基引发不饱和烃的串联反应[J]. 有机化学, 2022, 42(9): 2715-2727. |
| [7] | 赵晓正, 凌琴琴, 曹桂妍, 火星, 赵小龙, 苏瀛鹏. 炔丙醇类化合物参与的环化反应研究进展[J]. 有机化学, 2022, 42(9): 2605-2639. |
| [8] | 王苛莉, 黄静, 刘伟, 伍智林, 于贤勇, 蒋俊, 何卫民. 由N-(2-丙炔基)苯胺和磺酰氯直接合成3-砜基喹啉[J]. 有机化学, 2022, 42(8): 2527-2534. |
| [9] | 张文生, 李焱, 崔海燕, 苏小莉, 徐素鹏. 邻甲酰基苯甲酸甲酯还原胺化/内酰胺化一锅法合成N-取代异吲哚-1-酮[J]. 有机化学, 2022, 42(8): 2456-2461. |
| [10] | 周旭煜, 张爱君, 张庆庆, 刘庆安, 宣俊. 可见光诱导4-色满酮合成: 醋酸碘苯促进的α-酮酸与邻-烯丙氧基芳醛的自由基串联环化反应[J]. 有机化学, 2022, 42(8): 2488-2495. |
| [11] | 侯金松, 杨高升. 三(邻二甲胺基苄基)钇催化脂肪胺对烯腈的插入串联反应[J]. 有机化学, 2022, 42(7): 2070-2078. |
| [12] | 孙鑫, 屈超凡, 马超蕊, 赵筱薇, 柴国璧, 江智勇. 光氧化还原催化串联自由基加成反应构建1,4-二酮官能团化喹喔啉-2(1H)-酮衍生物[J]. 有机化学, 2022, 42(5): 1396-1406. |
| [13] | 肖立伟, 刘光仙, 任萍, 吴彤桐, 卢玉伟, 孔洁. 单质硫: 合成含硫杂环的优质硫源[J]. 有机化学, 2022, 42(4): 1002-1012. |
| [14] | 乔辉杰, 杨利婷, 陈雅, 王嘉琳, 孙武轩, 董昊博, 王云威. 温和条件下高效合成咪唑并杂环-肼类衍生物的三组分串联反应[J]. 有机化学, 2022, 42(4): 1188-1197. |
| [15] | 罗享豪, 谢益碧, 黄年玉, 王龙. 基于原位捕获异腈的Ugi四组分反应及其后修饰串联反应: 一锅法合成含氮杂环化合物[J]. 有机化学, 2022, 42(3): 838-846. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||