有机化学 ›› 2022, Vol. 42 ›› Issue (7): 2045-2054.DOI: 10.6023/cjoc202202025 上一篇 下一篇
所属专题: 有机氟化学虚拟合辑
综述与进展
收稿日期:2022-02-12
修回日期:2022-03-13
发布日期:2022-08-09
通讯作者:
张成潘
基金资助:Received:2022-02-12
Revised:2022-03-13
Published:2022-08-09
Contact:
Chengpan Zhang
Supported by:文章分享

含氟化合物的合成离不开含氟试剂的开发和应用. 由于氟原子的特殊效应, 含氟试剂通常表现出不同于不含氟类似物的反应性质和规律. 在众多三氟甲氧基化试剂中, 三氟甲磺酸三氟甲酯因具有制备简单、成本低廉及反应性质多样等优点, 近年来受到越来越多的关注. 该试剂可以在氟负离子引发下快速分解得到三氟甲氧基阴离子, 因此它常被看作是三氟甲氧基阴离子的储存体, 被广泛用于亲核三氟甲氧基化反应. 另外, 三氟甲磺酸三氟甲酯在亲核试剂引发下分解产生的三氟甲氧基阴离子, 由于其热稳定性较差, 很容易进一步分解成氟光气和氟负离子, 因此它还可以被用作羰基化试剂、氟甲酰化试剂、缩合试剂和亲核氟化试剂. 重点介绍了三氟甲磺酸三氟甲酯在三氟甲氧基化反应、羰基化反应以及氟化反应中的研究进展.
冉龙玉, 张成潘. 三氟甲磺酸三氟甲酯的反应研究进展[J]. 有机化学, 2022, 42(7): 2045-2054.
Longyu Ran, Chengpan Zhang. An Overview of the Reactions with Trifluoromethyl Trifluoromethanesulfonate[J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2045-2054.

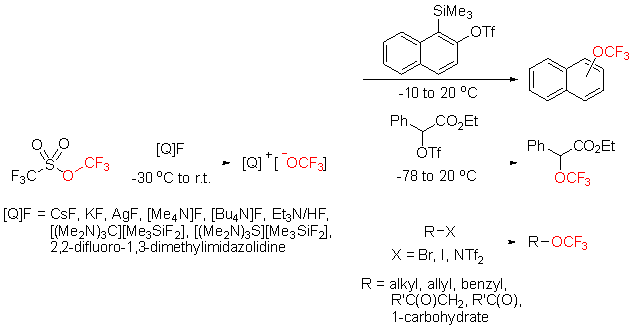
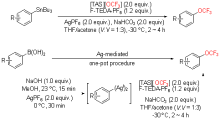
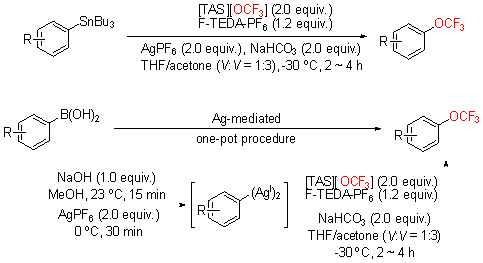
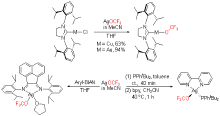
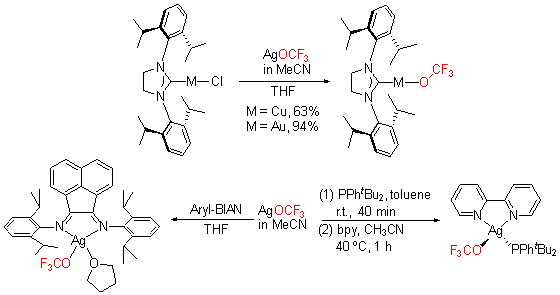
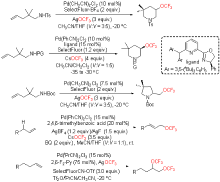

| [1] |
Selected books: (a) Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2013.
|
|
(b) Szabó, K. J.; Selander, N. Organofluorine Chemistry: Synthesis, Modeling, and Applications, WILEY-VCH GmbH, Weinheim, Germany, 2021.
|
|
|
(c) Ojima, I. Frontiers of Organofluorine Chemistry, World Scientific Publishing Europe Ltd., London WC2H 9HE, 2020.
|
|
|
(d) Ameduri, B.; Fomin, S. Fascinating Fluoropolymers and Their Applications, In Progress in Fluorine Science, 1st ed, Elsevier, Netherlands, 2020.
|
|
|
(e) Cahard, D.; Ma, J.-A. Emerging Fluorinated Motifs: Synthesis, Properties, and Applications, Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2020.
|
|
| [2] |
Selected reviews: (a) O'Hagan, D. Chem. Soc. Rev. 2008, 37, 308.
doi: 10.1039/b711844a pmid: 18197347 |
|
(b) Johnson, B. M.; Shu, Y.-Z.; Zhuo, X.; Meanwell, N. A. J. Med. Chem. 2020, 63, 6315.
doi: 10.1021/acs.jmedchem.9b01877 pmid: 18197347 |
|
|
(c) Alexandrino, D. A. M.; Mucha, A. P.; Almeida, C. M. R.; Carvalho, M. F. Crit. Rev. Biotechnol. 2021, https://doi.org/10.1080/07388551.2021.1977234.
pmid: 18197347 |
|
|
(d) Han, J.; Kiss, L.; Mei, H.; Remete, A. M.; Ponikvar-Svet, M.; Sedgwick, D. M.; Roman, R.; Fustero, S.; Moriwaki, H.; Soloshonok, V. A. Chem. Rev. 2021, 121, 4678.
doi: 10.1021/acs.chemrev.0c01263 pmid: 18197347 |
|
|
(e) Mondal, R.; Agbaria, M.; Nairoukh, Z. Chem.-Eur. J. 2021, 27, 7193.
doi: 10.1002/chem.202005425 pmid: 18197347 |
|
| [3] |
Selected reviews: (a) Inoue, M.; Sumii, Y.; Shibata, N. ACS Omega 2020, 5, 10633.
doi: 10.1021/acsomega.0c00830 |
|
(b) Bhutani, P.; Joshi, G.; Raja, N.; Bachhav, N.; Rajanna, P. K.; Bhutani, H.; Paul, A. T.; Kumar, R. J. Med. Chem. 2021, 64, 2339.
doi: 10.1021/acs.jmedchem.0c01786 |
|
|
(c) Zhang, M.; Li, S.; Zhang, H.; Xu, H. Eur. J. Med. Chem. 2020, 205, 112629.
doi: 10.1016/j.ejmech.2020.112629 |
|
|
(d) Jaye, J. A.; Sletten, E. M. Polym. Chem. 2021, 12, 6515.
doi: 10.1039/D1PY01024G |
|
| [4] |
Selected reviews: (a) Pons, A.; Delion, L.; Poisson, T.; Charette, A. B.; Jubault, P. Acc. Chem. Res. 2021, 54, 2969.
doi: 10.1021/acs.accounts.1c00261 pmid: 11848810 |
|
(b) Xiao, P.; Pannecoucke, X.; Bouillon, J.-P.; Couve-Bonnaire, S. Chem. Soc. Rev. 2021, 50, 6094.
doi: 10.1039/d1cs00216c pmid: 11848810 |
|
|
(c) Aggarwal, T.; Sushmita; Verma, A. K. Org. Chem. Front. 2021, 8, 6452.
doi: 10.1039/D1QO00952D pmid: 11848810 |
|
|
(d) Varga, B.; Csenki, J. T.; Tóth, B. L.; Béke, F.; Novák, Z.; Kotschy, A. Synthesis 2021, 53, 4313.
doi: 10.1055/a-1538-8344 pmid: 11848810 |
|
|
(e) Wang, S.-M.; Han, J.-B.; Zhang, C.-P.; Qin, H.-L.; Xiao, J.-C. Tetrahedron 2015, 71, 7949.
doi: 10.1016/j.tet.2015.06.056 pmid: 11848810 |
|
|
(f) Charpentier, J.; Früh, N.; Togni, A. Chem. Rev. 2015, 115, 650.
doi: 10.1021/cr500223h pmid: 11848810 |
|
|
(g) Umemoto, T. Chem. Rev. 1996, 96, 1757.
pmid: 11848810 |
|
|
(h) Yang, J.; Zhao, H.-W.; He, J.; Zhang, C.-P. Catalysts 2018, 8, 23.
doi: 10.3390/catal8010023 pmid: 11848810 |
|
| [5] |
Taylor, S. L.; Martin, J. C. J. Org. Chem. 1987, 52, 4147.
doi: 10.1021/jo00228a001 |
| [6] |
Kobayashi, Y.; Yoshida, T.; Kumadaski, I. Tetrahedron Lett. 1979, 40, 3865.
|
| [7] |
Noftle, R. E.; Cady, G. H. Inorg. Chem. 1965, 4, 1010.
doi: 10.1021/ic50029a019 |
| [8] |
(a) Olah, G. A.; Ohayama, T. Synthesis 1976, 319.
|
|
(b) Noftle, R. E. Inorg. Nucl. Chem. Lett. 1980, 16, 195.
doi: 10.1016/0020-1650(80)80120-6 |
|
| [9] |
Engelbrecht, V. A.; Tschager, E. Z. Anorg. Allg. Chem. 1977, 433, 19.
doi: 10.1002/zaac.19774330103 |
| [10] |
Katsuhara, Y.; DesMarteau, D. D. J. Am. Chem. Soc. 1980, 102, 2681.
doi: 10.1021/ja00528a027 |
| [11] |
(a) Oudrhiri-Hassani, M.; Germain, A.; Brunei, D.; Commeyras, A. Tetrahedron Lett. 1981, 22, 65.
doi: 10.1016/0040-4039(81)80042-1 |
|
(b) Oudrhiri-Hassani, M.; Brunel, D.; Germain, A.; Commeyras, A. J. Fluorine Chem. 1984, 25, 219.
doi: 10.1016/S0022-1139(00)80951-3 |
|
| [12] |
Song, H.-X.; Tian, Z.-Y.; Xiao, J.-C.; Zhang, C.-P. Chem.-Eur. J. 2020, 26, 16261.
doi: 10.1002/chem.202003756 |
| [13] |
(a) Lin, J.-H.; Ji, Y.-L.; Xiao, J.-C. Curr. Org. Chem. 2015, 19, 1541.
doi: 10.2174/1385272819666150520230727 |
|
(b) Tlili, A.; Toulgoat, F.; Billard, T. Angew. Chem., Int. Ed. 2016, 55, 11726.
doi: 10.1002/anie.201603697 |
|
|
(c) Lee, K. N.; Lee, J. W.; Ngai, M.-Y. Synlett 2016, 27, 313.
doi: 10.1055/s-0035-1560516 |
|
|
(d) Besset, T.; Jubault, P.; Pannecoucke, X.; Poisson, T. Org. Chem. Front. 2016, 3, 1004.
doi: 10.1039/C6QO00164E |
|
|
(e) Lee, K. N.; Lee, J. W.; Ngai, M.-Y. Tetrahedron 2018, 74, 7127.
doi: 10.1016/j.tet.2018.09.020 |
|
|
(f) Lee, J. W.; Lee, K. N.; Ngai, M.-Y. Angew. Chem., Int. Ed. 2019, 58, 11171.
doi: 10.1002/anie.201902243 |
|
|
(g) Zhang, X.; Tang, P. Sci. China: Chem. 2019, 62, 525.
|
|
|
(h) Jiang, X.; Tang, P. Chin. J. Chem. 2021, 39, 255.
doi: 10.1002/cjoc.202000465 |
|
|
(i) Wang, Q.; Zhang, X.; Sorochinsky, A. E.; Butler, G.; Han, J.; Soloshonok, V. A. Symmetry 2021, 13, 2380.
doi: 10.3390/sym13122380 |
|
| [14] |
(a) Umemoto, T.; Adachi, K.; Ishihara, S. J. Org. Chem. 2007, 72, 6905.
pmid: 17676906 |
|
(b) Koller, R.; Stanek, K.; Stolz, D.; Aardoom, R.; Niedermann, K.; Togni, A. Angew. Chem., Int. Ed. 2009, 48, 4332.
doi: 10.1002/anie.200900974 pmid: 17676906 |
|
|
(c) Koller, R.; Huchet, Q.; Battaglia, P.; Welch, J. M.; Togni, A. Chem. Commun. 2009, 5993.
pmid: 17676906 |
|
|
(d) Liang, A.; Han, S.; Liu, Z.; Wang, L.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Chem.-Eur. J. 2016, 22, 5102.
doi: 10.1002/chem.201505181 pmid: 17676906 |
|
|
(e) Hojczyk, K. N.; Feng, P.; Zhan, C.; Ngai, M.-Y. Angew. Chem., Int. Ed. 2014, 53, 14559.
doi: 10.1002/anie.201409375 pmid: 17676906 |
|
|
(f) Feng, P.; Lee, K. N.; Lee, J. W.; Zhan, C.; Ngai, M.-Y. Chem. Sci. 2016, 7, 424.
doi: 10.1039/C5SC02983J pmid: 17676906 |
|
|
(g) Liu, J.-B.; Chen, C.; Chu, L.; Chen, Z.-H.; Xu, X.-H.; Qing, F.-L. Angew. Chem., Int. Ed. 2015, 54, 11839.
doi: 10.1002/anie.201506329 pmid: 17676906 |
|
|
(h) Liu, J.-B.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2015, 17, 5048.
doi: 10.1021/acs.orglett.5b02522 pmid: 17676906 |
|
|
(i) Ouyang, Y.; Xu, X.-H.; Qing, F.-L. Angew. Chem., Int. Ed. 2022, 61, e202114048.
pmid: 17676906 |
|
| [15] |
(a) Zhou, M.; Ni, C.; He, Z.; Hu, J. Org. Lett. 2016, 18, 3754.
doi: 10.1021/acs.orglett.6b01779 pmid: 27560791 |
|
(b) Zhang, Q.-W.; Brusoe, A. T.; Mascitti, V.; Hesp, K. D.; Blakemore, D. C.; Kohrt, J. T.; Hartwig, J. F. Angew. Chem., Int. Ed. 2016, 55, 9758.
doi: 10.1002/anie.201604793 pmid: 27560791 |
|
|
(c) Chatalova-Sazepin, C.; Binayeva, M.; Epifanov, M.; Zhang, W.; Foth, P.; Amador, C.; Jagdeo, M.; Boswell, B. R.; Sammis, G. M. Org. Lett. 2016, 18, 4570.
doi: 10.1021/acs.orglett.6b02208 pmid: 27560791 |
|
| [16] |
(a) Allison, J. A. C.; Cady, G. H. J. Am. Chem. Soc. 1959, 81, 1089.
doi: 10.1021/ja01514a018 |
|
(b) Venturini, F.; Navarrini, W.; Famulari, A.; Sansotera, M.; Dardani, P.; Tortelli, V. J. Fluorine Chem. 2012, 140, 43.
doi: 10.1016/j.jfluchem.2012.04.008 |
|
|
(c) Johri, K. K.; DesMarteau, D. D. J. Org. Chem. 1983, 48, 242.
doi: 10.1021/jo00150a019 |
|
|
(d) Navarrini, W.; Venturini, F.; Sansotera, M.; Ursini, M.; Metrangolo, P.; Resnati, G.; Galimberti, M.; Barchiesi, E.; Dardani, P. J. Fluorine Chem. 2008, 129, 680.
doi: 10.1016/j.jfluchem.2008.05.018 |
|
|
(e) Hesse, R. H. Trifluoromethyl Hypofluorite, e-EROS Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, Ltd., 2001.
|
|
| [17] |
(a) Zheng, W.; Morales-Rivera, C. A.; Lee, J. W.; Liu, P.; Ngai, M.-Y. Angew. Chem., Int. Ed. 2018, 57, 9645.
doi: 10.1002/anie.201800598 |
|
(b) Zheng, W.; Lee, J. W.; Morales-Rivera, C. A.; Liu, P.; Ngai, M.-Y. Angew. Chem., Int. Ed. 2018, 57, 13795.
doi: 10.1002/anie.201808495 |
|
|
(c) Lee, J. W.; Lim, S.; Maienshein, D. N.; Liu, P.; Ngai, M.-Y. Angew. Chem., Int. Ed. 2020, 59, 21475.
doi: 10.1002/anie.202009490 |
|
|
(d) Nyuchev, A. V.; Wan, T.; Cendón, B.; Sambiagio, C.; Struijs, J. J. C.; Ho, M.; Gulías, M.; Wang, Y.; Noël, T. Beilstein J. Org. Chem. 2020, 16, 1305.
doi: 10.3762/bjoc.16.111 |
|
|
(e) Duhail, T.; Bortolato, T.; Mateos, J.; Anselmi, E.; Jelier, B.; Togni, A.; Magnier, E.; Dagousset, G.; Dell’Amico, L. Org. Lett. 2021, 23, 7088.
doi: 10.1021/acs.orglett.1c02494 |
|
|
(f) Jelier, B. J.; Tripet, P. F.; Pietrasiak, E.; Franzoni, I.; Jeschke, G.; Togni, A. Angew. Chem., Int. Ed. 2018, 57, 13784.
doi: 10.1002/anie.201806296 |
|
|
(g) Dix, S.; Golz, P.; Schmid, J. R.; Riedel, S.; Hopkinson, M. N. Chem.-Eur. J. 2021, 27, 11554.
doi: 10.1002/chem.202101621 |
|
| [18] |
Billard, T. Methanesulfonic Acid, 1,1,1-Trifluoro-, Trifluoromethyl Ester, e-EROS Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, Ltd., 2016, pp. 1-3.
|
| [19] |
Lu, Z.; Kumon, T.; Hammond, G. B.; Umemoto, T. Angew. Chem., Int. Ed. 2021, 60, 16171.
doi: 10.1002/anie.202104975 |
| [20] |
(a) Marrec, O.; Billard, T.; Vors, J.-P.; Pazenok, S.; Langlois, B. R. Adv. Synth. Catal. 2010, 352, 2831.
doi: 10.1002/adsc.201000488 |
|
(b) Bonnefoy, C.; Chefdeville, E.; Panosian, A.; Hanquet, G.; Leroux, F. R.; Toulgoat, F.; Billard, T. Chem.-Eur. J. 2021, 27, 15986.
doi: 10.1002/chem.202102809 |
|
|
(c) Duran-Camacho, G.; Ferguson, D. M.; Kampf, J. W.; Bland, D. C.; Sanford, M. S. Org. Lett. 2021, 23, 5138.
doi: 10.1021/acs.orglett.1c01664 |
|
| [21] |
(a) Guo, S.; Cong, F.; Guo, R.; Wang, L.; Tang, P. Nat. Chem. 2017, 9, 546.
doi: 10.1038/nchem.2711 |
|
(b) Jiang, X.; Deng, Z.; Tang, P. Angew. Chem., Int. Ed. 2018, 57, 292.
doi: 10.1002/anie.201711050 |
|
|
(c) Yang, H.; Wang, F.; Jiang, X.; Zhou, Y.; Xu, X.; Tang, P. Angew. Chem., Int. Ed. 2018, 57, 13266.
doi: 10.1002/anie.201807144 |
|
|
(d) Liu, J.; Wei, Y.; Tang, P. J. Am. Chem. Soc. 2018, 140, 15194.
doi: 10.1021/jacs.8b10298 |
|
|
(e) Wang, F.; Xu, P.; Cong, F.; Tang, P. Chem. Sci. 2018, 9, 8836.
doi: 10.1039/C8SC03730B |
|
|
(f) Cong, F.; Wei, Y.; Tang, P. Chem. Commun. 2018, 54, 4473.
doi: 10.1039/C8CC01096J |
|
|
(g) Yang, S.; Chen, M.; Tang, P. Angew. Chem., Int. Ed. 2019, 58, 7840.
doi: 10.1002/anie.201901447 |
|
|
(h) Huang, Q.; Tang, P. J. Org. Chem. 2020, 85, 2512.
doi: 10.1021/acs.joc.9b03206 |
|
|
(i) Jiang, X.; Tang, P. Org. Lett. 2020, 22, 5135.
doi: 10.1021/acs.orglett.0c01741 |
|
|
(j) Deng, Z.; Zhao, M.; Wang, F.; Tang, P. Nat. Commun. 2020, 11, 2569.
doi: 10.1038/s41467-020-16451-x |
|
|
(k) Wang, F.; Guo, Y.; Zhang, Y.; Tang, P. ACS Catal. 2021, 11, 3218.
doi: 10.1021/acscatal.1c00090 |
|
|
(l) Xin, J.; Deng, X.; Tang, P. Org. Lett. 2022, 24, 881.
doi: 10.1021/acs.orglett.1c04226 |
|
| [22] |
Li, Y.; Yang, Y.; Xin, J.; Tang, P. Nat. Commun. 2020, 11, 755.
doi: 10.1038/s41467-020-14598-1 pmid: 32029731 |
| [23] |
Zhou, M.; Ni, C.; Zeng, Y.; Hu, J. J. Am. Chem. Soc. 2018, 140, 6801.
doi: 10.1021/jacs.8b04000 pmid: 29787259 |
| [24] |
Kolomeitsev, A. A.; Vorobyev, M.; Gillandt, H. Tetrahedron Lett. 2008, 49, 449.
doi: 10.1016/j.tetlet.2007.11.105 |
| [25] |
(a) Marrec, O.; Billard, T.; Vors, J.-P.; Pazenok, S.; Langlois, B. R. J. Fluorine Chem. 2010, 131, 200.
doi: 10.1016/j.jfluchem.2009.11.006 |
|
(b) Sokolenko, T. M.; Davydova, Y. A.; Yagupolskii, Y. L. J. Fluorine Chem. 2012, 136, 20.
doi: 10.1016/j.jfluchem.2012.01.005 |
|
| [26] |
Barbion, J.; Pazenok, S.; Vors, J.-P.; Langlois, B. R.; Billard, T. Org. Proc. Res. Dev. 2014, 18, 1037.
|
| [27] |
Huang, C.; Liang, T.; Harada, S.; Lee, E.; Ritter, T. J. Am. Chem. Soc. 2011, 133, 13308.
doi: 10.1021/ja204861a |
| [28] |
Zhang, C.-P.; Vicic, D. A. Organometallics 2012, 31, 7812.
doi: 10.1021/om3002747 |
| [29] |
Chen, S.; Huang, Y.; Fang, X.; Li, H.; Zhang, Z.; Horb, T. S. A.; Weng, Z. Dalton Trans. 2015, 44, 19682.
doi: 10.1039/C5DT02078F |
| [30] |
Chen, D.; Lu, L.; Shen, Q. Org. Chem. Front. 2019, 6, 1801.
doi: 10.1039/C9QO00278B |
| [31] |
(a) Chen, C.-H.; Chen, P.-H.; Liu, G.-S. J. Am. Chem. Soc. 2015, 137, 15648.
doi: 10.1021/jacs.5b10971 |
|
(b) Chen, C.-H.; Pfluger, P. M.; Chen, P.-H.; Liu, G.-S. Angew. Chem., Int. Ed. 2019, 58, 2392.
doi: 10.1002/anie.201813591 |
|
|
(c) Chen, C.-H.; Hou, C.-Q.; Chen, P.-H.; Liu, G.-S. Chin. J. Chem. 2020, 38, 346.
doi: 10.1002/cjoc.201900516 |
|
|
(d) Qi, X.-X.; Chen, P.-H.; Liu, G.-S. Angew. Chem., Int. Ed. 2017, 56, 9517.
doi: 10.1002/anie.201703841 |
|
|
(e) Chen, C.-H.; Luo, Y.-X.; Fu, L.; Chen, P.-H.; Lan, Y.; Liu, G.-S. J. Am. Chem. Soc. 2018, 140, 1207.
doi: 10.1021/jacs.7b11470 |
|
| [32] |
Zha, G.-F.; Han, J.-B.; Hu, X.-Q.; Qin, H.-L.; Fang, W.-Y.; Zhang, C.-P. Chem. Commun. 2016, 52, 7458.
doi: 10.1039/C6CC03040H |
| [33] |
Zhang, Q.-W.; Hartwig, J. F. Chem. Commun. 2018, 54, 10124.
doi: 10.1039/C8CC05084H |
| [34] |
Zhang, W.; Chen, J.; Lin, J.-H.; Xiao, J.-C.; Gu, Y.-C. iScience 2018, 5, 110.
doi: S2589-0042(18)30092-0 pmid: 30240641 |
| [35] |
Yang, Y.-M.; Yao, J.-F.; Yan, W.; Luo, Z.; Tang, Z.-Y. Org. Lett. 2019, 21, 8003.
doi: 10.1021/acs.orglett.9b03000 |
| [36] |
(a) Saiter, J.; Guérin, T.; Donnard, M.; Panossian, A.; Hanquet, G.; Leroux, F. R. Eur. J. Org. Chem. 2021, 3139.
|
|
(b) Farnham, W. B.; Smart, B. E.; Middleton, W. J.; Calabrese, J. C.; Dixon, D. A. J. Am. Chem. Soc. 1985, 107, 4565.
doi: 10.1021/ja00301a043 |
|
|
(c) Trainor, G. L. J. Carbohydr. Chem. 1985, 4, 545.
doi: 10.1080/07328308508082676 |
|
|
(d) Yu, J.; Lin, J.-H.; Yu, D.; Du, R.; Xiao, J.-C. Nat. Commun. 2019, 10, 5362.
doi: 10.1038/s41467-019-13359-z |
|
|
(e) Newton, J. J.; Jelier, B. J.; Meanwell, M.; Martin, R. E.; Britton, R.; Friesen, C. M. Org. Lett. 2020, 22, 1785.
doi: 10.1021/acs.orglett.0c00099 |
|
| [37] |
(a) Delebecq, E.; Pascault, J.-P.; Boutevin, B.; Ganachaud, F. Chem. Rev. 2013, 113, 80.
doi: 10.1021/cr300195n pmid: 23082894 |
|
(b) Baars, H.; Engel, J.; Mertens, L.; Meister, D.; Bolm, C. Adv. Synth. Catal. 2016, 358, 2293.
doi: 10.1002/adsc.201600308 pmid: 23082894 |
|
| [38] |
Quan, H.; Zhang, N.; Zhou, X.; Qian, H.; Sekiya, A. J. Fluorine Chem. 2015, 176, 26.
doi: 10.1016/j.jfluchem.2015.05.007 |
| [39] |
Song, H.-X.; Han, Z.-Z.; Zhang, C.-P. Chem.-Eur. J. 2019, 25, 10907.
doi: 10.1002/chem.201901865 |
| [40] |
(a) Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004.
|
|
(b) J, Dong.; L, Krasnova.; M, G. Finn.; Sharpless, K. B. Angew. Chem., Int. Ed. 2014, 53, 9430.
doi: 10.1002/anie.201309399 |
|
| [41] |
Ogiwara, Y.; Sakai, N. Angew. Chem., Int. Ed. 2020, 59, 574.
doi: 10.1002/anie.201902805 |
| [1] | 李洋, 董亚楠, 李跃辉. 经由N-硼基酰胺中间体的酰胺高效转化合成腈类化合物[J]. 有机化学, 2024, 44(2): 638-643. |
| [2] | 金玉坤, 任保轶, 梁福顺. 可见光介导的三氟甲基的选择性C-F键断裂及其在偕二氟类化合物合成中的应用[J]. 有机化学, 2024, 44(1): 85-110. |
| [3] | 朱传涛, 王松, 赵一凡, Herdewijn Piet, 刘丰五. 新型2,2'-缩水-L-苏糖嘧啶膦酸核苷的合成[J]. 有机化学, 2023, 43(9): 3167-3173. |
| [4] | 席敏, 段超, 迟捷, 付甜, 苏小龙, 王宏社. 腐殖酸作用下Strecker反应快速高效合成α-氨基腈[J]. 有机化学, 2023, 43(9): 3312-3318. |
| [5] | 吴文倩, 陈春霞, 彭进松, 李占宇. 羰基α-位胺化反应研究进展[J]. 有机化学, 2023, 43(8): 2743-2763. |
| [6] | 安大列, 包志鹏, 吴小锋. 含碳氟类底物参与的羰基化反应研究进展[J]. 有机化学, 2023, 43(7): 2304-2312. |
| [7] | 曾成富, 何媛, 李清, 董琳. Ir(III)催化新型三组分串联三氟乙氧基化反应并一锅法构建复杂酰胺化合物[J]. 有机化学, 2023, 43(3): 1115-1123. |
| [8] | 曹伟地, 刘小华. 不对称催化质子化构建α-叔碳羰基化合物研究进展[J]. 有机化学, 2023, 43(3): 961-973. |
| [9] | 张建涛, 邓雅文, 莫诺琳, 陈莲芬. 自由基介导的α,α-二芳基烯丙醇1,2-芳基迁移反应研究进展[J]. 有机化学, 2023, 43(2): 426-435. |
| [10] | 郭广青, 练仲. 硅基羧酸在有机合成中的应用进展[J]. 有机化学, 2023, 43(10): 3580-3589. |
| [11] | 张豪, 赵庆彬, 阮忠睿, 刘振兴. 硅醚与硫(VI)氟化合物SuFEx点击反应进展[J]. 有机化学, 2023, 43(10): 3569-3579. |
| [12] | 孙奇, 孙泽颖, 俞泽, 王光伟. 镍催化炔烃的立体选择性芳基-二氟烷基化反应[J]. 有机化学, 2022, 42(8): 2515-2520. |
| [13] | 李响, 张依凡, 陆凯琳, 刘石惠, 张永强. 基于莪术醇胺氟化结构修饰的三维天然产物片段库的构建[J]. 有机化学, 2022, 42(7): 2124-2133. |
| [14] | 马志伟, 陈晓培, 王川川, 王建玲, 陶京朝, 吕全建. 手性方酰胺催化环状1,3-二羰基化合物对β,γ-不饱和-α-酮酯的不对称Michael加成反应[J]. 有机化学, 2022, 42(5): 1520-1526. |
| [15] | 韩高旭, 许红涛, 侯卫. 铑(III)催化的C(sp3)—H官能团化[J]. 有机化学, 2022, 42(2): 391-423. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
