有机化学 ›› 2022, Vol. 42 ›› Issue (7): 2000-2014.DOI: 10.6023/cjoc202202028 上一篇 下一篇
综述与进展
王亚洲a, 祝宇航b, 徐丽霞c, 贺瑞c,*( ), 张坚b,c,*(
), 张坚b,c,*( )
)
收稿日期:2022-02-23
修回日期:2022-03-23
发布日期:2022-08-09
通讯作者:
贺瑞, 张坚
基金资助:
Yazhou Wanga, Yuhang Zhub, Lixia Xuc, Rui Hec( ), Jian Zhangb,c(
), Jian Zhangb,c( )
)
Received:2022-02-23
Revised:2022-03-23
Published:2022-08-09
Contact:
Rui He, Jian Zhang
Supported by:文章分享
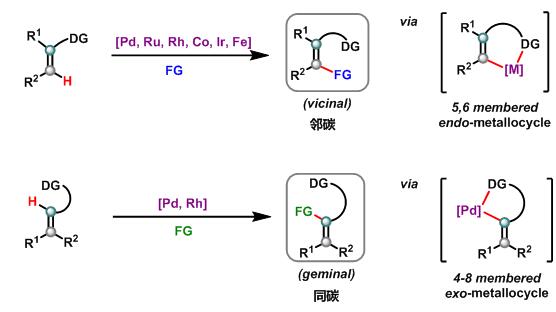
配位导向下烯基C—H官能团化是制备多取代烯烃的常用方法, 反应通常经历弱配位基团(羟基、酰胺、羧酸、胺基等)协助下的双键在环内的金属杂环中间体(endo-metallocycle), 实现导向基邻碳位C—H官能团化, 表现出了优异的Z/E选择性, 目前发展的C—H官能团化类型丰富, 包括烯基化、烷基化、芳基化、炔基化、烯丙基化、杂原子化和多米诺环化等. 相比较而言, 导向基同碳位烯基C—H官能团化则发展缓慢, 可能是该反应需要经历更加不稳定的双键在环外的金属杂环中间体(exo-metallocycle), 严重阻碍了烯烃的配位C—H活化的发展. 近年来, 若干课题组在这方面报道了导向基同碳位烯基C—H官能团化, 包括烯基化、烯丙基化、芳基化、碘代、炔基化反应. 按C—H官能化类型介绍了配位导向下同碳位烯基C—H官能团化反应的研究进展, 并对该领域的发展前景进行了总结与展望.
王亚洲, 祝宇航, 徐丽霞, 贺瑞, 张坚. 配位导向下烯基同碳位C—H官能团化反应进展[J]. 有机化学, 2022, 42(7): 2000-2014.
Yazhou Wang, Yuhang Zhu, Lixia Xu, Rui He, Jian Zhang. Recent Advances in Geminal-Group-Directed Alkenyl C—H Functionalization[J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2000-2014.
| [1] |
For selected reviews on the application of C-H activation, see: (a) Abrams, D. J.; Provencher, P. A.; Sorensen, E. J. Chem. Soc. Rev. 2018, 47, 8925.
doi: 10.1039/c8cs00716k pmid: 25144592 |
|
(b) Noisier, A. F.; Brimble, M. A. Chem. Rev. 2014, 114, 8775.
doi: 10.1021/cr500200x pmid: 25144592 |
|
|
(c) Baccalini, A.; Faita, G.; Zanoni, G.; Maiti, D. Chem.-Eur. J. 2020, 26, 9749.
doi: 10.1002/chem.202001832 pmid: 25144592 |
|
|
(d) Ren, Q.; Nie, B.; Zhang, Y.; Zhang, J. Chin. J. Org. Chem. 2018, 38, 2465. (in Chinese)
doi: 10.6023/cjoc201803002 pmid: 25144592 |
|
|
( 任青云, 聂飚, 张英俊, 张霁, 有机化学, 2018, 38, 2465.)
pmid: 25144592 |
|
|
(e) Sinha, S. K.; Zanoni, G.; Maiti, D. Asian J. Org. Chem. 2018, 7, 1178.
doi: 10.1002/ajoc.201800203 pmid: 25144592 |
|
| [2] |
(a) Murahashi, S. J. Am. Chem. Soc. 1955, 77, 6403.
|
|
(b) Fahey, D. R. J. Organomet. Chem. 1971, 27, 283.
doi: 10.1016/S0022-328X(00)80577-X |
|
|
(c) Lewis, L. N.; Smith, J. F. J. Am. Chem. Soc. 1986, 108, 2728.
doi: 10.1021/ja00270a036 |
|
| [3] |
Murai, S.; Kakiuchi, F.; Sekine, S.; Tanaka, Y.; Kamatani, A.; Sonoda, M.; Chatani, N. Nature 1993, 366, 529.
doi: 10.1038/366529a0 |
| [4] |
(a) Sambiagio, C.; Schönbauer, D.; Blieck, R.; DaoHuy, T.; Pototschnig, G.; Schaaf, P.; Wiesinger, T.; Zia, M. F.; Wencel-Delord, J.; Besset, T.; Maes, B. U. W.; Schnürch, M. Chem. Soc. Rev. 2018, 47, 6603.
doi: 10.1039/c8cs00201k pmid: 30033454 |
|
(b) Rej, S.; Ano, Y.; Chatani, N. Chem. Rev. 2020, 120, 1788.
doi: 10.1021/acs.chemrev.9b00495 pmid: 30033454 |
|
| [5] |
(a) Yu, M.; Xie, Y.; Xie, C.; Zhang, Y. Org. Lett. 2012, 14, 2164.
doi: 10.1021/ol3006997 |
|
(b) Yao, J.; Yu, M.; Zhang, Y. Adv. Synth. Catal. 2012, 354, 3205.
doi: 10.1002/adsc.201200447 |
|
|
(c) Zhang, X.-S.; Zhu, Q.-L.; Zhang, Y.-F.; Li, Y.-B.; Shi, Z.-J. Chem.-Eur. J. 2013, 19, 11898.
doi: 10.1002/chem.201300829 |
|
| [6] |
(a) Unoh, Y.; Satoh, T.; Hirano, K.; Miura, M. ACS Catal. 2015, 5, 6634.
doi: 10.1021/acscatal.5b01896 |
|
(b) Liu, Z.; Wu, J.-Q.; Yang, S.-D. Org. Lett. 2017, 19, 5434.
doi: 10.1021/acs.orglett.7b02710 |
|
|
(c) Yang, Y.; Qiu, X.; Zhao, Y.; Mu, Y.. Z. Shi, J. Am. Chem. Soc. 2016, 138, 495.
|
|
| [7] |
(a) Murai, M.; Matsumoto, K.; Takeuchi, Y.; Takai, K. Org. Lett. 2015, 17, 3102.
doi: 10.1021/acs.orglett.5b01373 |
|
(b) Zhang, Q.-W.; An, K.; Liu, L.-C.; Yue, Y.; He, W. Angew. Chem., Int. Ed. 2015, 54, 6918.
doi: 10.1002/anie.201502548 |
|
|
(c) Simmons, E. M.; Hartwig, J. F. J. Am. Chem. Soc. 2010, 132, 17092.
doi: 10.1021/ja1086547 |
|
| [8] |
Wang, J. Stereoselective Alkene Synthesis, Springer Verlag, Heidelberg, New York, 2012.
|
| [9] |
Tang, S.; Liu, K.; Liu, C.; Lei, A. Chem. Soc. Rev. 2015, 44, 1070.
doi: 10.1039/C4CS00347K |
| [10] |
(a) Shang, X.; Liu, Z.-Q. Chem. Soc. Rev. 2013, 42, 3253.
doi: 10.1039/c2cs35445d pmid: 33491691 |
|
(b) Zhang, J.; Lu, X.; Shen, C.; Xu, L.; Ding, L.; Zhong, G. Chem. Soc. Rev. 2021, 50, 3263.
doi: 10.1039/d0cs00447b pmid: 33491691 |
|
| [11] |
Liu, B.; Yang, L.; Li, P.; Wang, F.; Li, X. Org. Chem. Front. 2021, 8, 1085.
doi: 10.1039/D0QO01159B |
| [12] |
Examples for Rh-catalysis: (a) Besset, T.; Kuhl, N.; Patureau., F. W.; Glorius, F. Chem.-Eur. J. 2011, 17, 7167.
doi: 10.1002/chem.201101340 |
|
(b) Zhang, J.; Loh, T.-P. Chem. Commun. 2012, 48, 11232.
doi: 10.1039/c2cc36137j |
|
|
For, Ru-catalysis.
|
|
|
(c) Li., F., Yu, C.; Zhang, J.; Zhong, G. Org. Lett. 2016, 18, 4582.
doi: 10.1021/acs.orglett.6b02229 |
|
|
(d) Li, T.; Zhang, J.; Yu, C.; Lu, X.; Xu, L.; Zhong, G. Chem. Commun. 2017, 53, 12926.
doi: 10.1039/C7CC07777G |
|
|
For Pd-catalysis: (e) Liang, Q.-J.; Yang, C.; Meng, F.-F.; Jiang, B.; Xu, Y.-H.; Loh, T.-P. Angew. Chem., Int. Ed. 2017, 56, 5091.
doi: 10.1002/anie.201700559 |
|
|
(f) Parella, R.; Babu, S. A. J. Org. Chem. 2015, 80, 12379.
doi: 10.1021/acs.joc.5b02264 |
|
|
For Ir-catalysis : (g) Meng, K.; Sun, Y.; Zhang, J.; Zhang, K.; Ji, X.; Ding, L.; Zhong, G. Org. Lett. 2019, 21, 8219.
doi: 10.1021/acs.orglett.9b02935 |
|
|
(h) Xu, L.; Meng, K.; Zhang, J.; Sun, Y.; Lu, X.; Li, T.; Jiang, Y.; Zhong, G. Chem. Commun. 2019, 55, 9757.
doi: 10.1039/C9CC04419A |
|
|
(i) Huang, Y.; Xu, L.; Yu, F.; Shen, W.; Lu, X.; Ding, L.; Zhong, L.; Zhong, G.; Zhang, J. J. Org. Chem. 2020, 85, 7225.
doi: 10.1021/acs.joc.0c00619 |
|
|
For, Co-catalysis.
|
|
|
(j) Li, T.; Shen, C.; Sun, Y.; Zhang, J.; Xiang, P.; Lu, X.; Zhong, G. Org. Lett. 2019, 21, 7772.
doi: 10.1021/acs.orglett.9b02717 |
|
| [13] |
Mawo, R. Y., Mustakim, S., Young, V. G., Hoffmann, M. R.; Smoliakova, I. P. Organometallics 2007, 26, 1801.
doi: 10.1021/om061132p |
| [14] |
Liu, M.; Yang, P.; Karunananda, M. K.; Wang, Y.; Liu, P.; Engle, K. M. J. Am. Chem. Soc. 2018, 140, 5805.
doi: 10.1021/jacs.8b02124 |
| [15] |
Xu, S.; Hirano, K.; Miura, M. Org. Lett. 2020, 22, 9059.
doi: 10.1021/acs.orglett.0c03444 |
| [16] |
Schreib, B. S.; Son, M.; Aouane, F. A.; Baik, M.-H.; Carreira, E. M. J. Am. Chem. Soc. 2021, 143, 21705.
doi: 10.1021/jacs.1c11528 |
| [17] |
Shen, C.; Zhu, Y.; Jin, S.; Xu, K.; Luo, S.; Xu, L.; Zhong, G.; Zhong, L.; Zhang, J. Org. Chem. Front. 2022, 9, 989.
doi: 10.1039/D1QO01676H |
| [18] |
Tsai, H.-C.; Huang, Y.-H.; Chou, C.-M. Org. Lett. 2018, 20, 1328.
doi: 10.1021/acs.orglett.8b00064 |
| [19] |
Wang, Y.-C.; Huang, Y.-H.; Tsai, H.-C.; Basha, R.-S.; Chou, C.-M. Org. Lett. 2020, 22, 6765.
doi: 10.1021/acs.orglett.0c02241 |
| [20] |
Meng, K.; Li, T.; Yu, C.; Shen, C.; Zhang, J.; Zhong, G. Nat. Commun. 2019, 10, 5109.
doi: 10.1038/s41467-019-13098-1 |
| [21] |
Zhu, Y.; Chen, F.; Cheng, D.; Chen, Y.; Zhao, X.; Wei, W.; Lu, Y.; Zhao, J. Org. Lett. 2020, 22, 8786.
doi: 10.1021/acs.orglett.0c03126 |
| [22] |
Shen, C.; Lu, X.; Zhang, J.; Ding, L.; Sun, Y.; Zhong, G. Chem. Commun. 2019, 55, 13582.
doi: 10.1039/C9CC07466J |
| [23] |
Han, B.; Li, B.; Qi, L.; Yang, P.; He, G.; Chen, G. Org. Lett. 2020, 22, 6879.
doi: 10.1021/acs.orglett.0c02422 |
| [24] |
Wu, Z.; Fatuzzo, N.; Dong, G. J. Am. Chem. Soc. 2020, 142, 2715.
doi: 10.1021/jacs.9b11479 |
| [25] |
Schreib, B. S.; Fadel, M.; Carreira, E. M. Angew. Chem., Int. Ed. 2020, 59, 7818.
doi: 10.1002/anie.202000935 |
| [26] |
Schreib, B. S.; Carreira, E. M. J. Am. Chem. Soc. 2019, 141, 8758.
doi: 10.1021/jacs.9b03998 |
| [1] | 付雅彤, 孙超凡, 张丹, 金成国, 陆居有. 巢式-碳硼烷硼氢键官能化反应研究进展[J]. 有机化学, 2024, 44(2): 438-447. |
| [2] | 李思达, 崔鑫, 舒兴中, 吴立朋. 钛催化的烯烃制备1,1-二硼化合物[J]. 有机化学, 2024, 44(2): 631-637. |
| [3] | 赵红琼, 于淼, 宋冬雪, 贾琦, 刘颖杰, 季宇彬, 许颖. 羧酸脱羧羟基化反应研究进展[J]. 有机化学, 2024, 44(1): 70-84. |
| [4] | 高晓阳, 翟锐锐, 陈训, 王烁今. 碳酸亚乙烯酯参与C—H键活化反应的研究进展[J]. 有机化学, 2023, 43(9): 3119-3134. |
| [5] | 张建涛, 张聪, 莫诺琳, 罗佳婷, 陈莲芬, 刘卫兵. 氯仿参与的烯烃自由基加成反应的研究进展[J]. 有机化学, 2023, 43(9): 3098-3106. |
| [6] | 陈新强, 张敬. 伯醇的脱羟甲基反应的研究进展[J]. 有机化学, 2023, 43(8): 2711-2719. |
| [7] | 董思凡, 李昊龙, 秦源, 范士明, 刘守信. 氨基酸作为瞬态导向基在碳氢键活化反应中的研究进展[J]. 有机化学, 2023, 43(7): 2351-2367. |
| [8] | 陆晓雨, 孙晓梅, 钮亚琴, 王俊超, 殷文婧, 高梦婷, 刘孜, 韦正桓, 陶庭骅. 铜催化氟代丙烯酸与氧杂吖丙啶的脱羧交叉偶联反应[J]. 有机化学, 2023, 43(6): 2110-2119. |
| [9] | 徐光利, 许静, 徐海东, 崔香, 舒兴中. 过渡金属催化烯烃和炔烃合成1,3-共轭二烯化合物研究进展[J]. 有机化学, 2023, 43(6): 1899-1933. |
| [10] | 户晓兢, 郭斐翔, 朱润青, 周柄棋, 张涛, 房立真. 对烷氧基酚的合成及其去芳构化后的合成应用[J]. 有机化学, 2023, 43(6): 2239-2244. |
| [11] | 卢凯, 屈浩琦, 陈樨, 秋慧, 郑晶, 马猛涛. 无催化剂、无溶剂条件下炔烃和烯烃与儿茶酚硼烷的硼氢化反应[J]. 有机化学, 2023, 43(6): 2197-2205. |
| [12] | 蒋旺, 史壮志. 芳烃间/对位选择性碳氢硼化反应研究进展[J]. 有机化学, 2023, 43(5): 1691-1705. |
| [13] | 李思达, 舒兴中, 吴立朋. 锆、钛介导的烯烃、炔烃硼氢化[J]. 有机化学, 2023, 43(5): 1751-1760. |
| [14] | 高师泉, 刘闯军, 杨俊锋, 张俊良. 钴催化的烯烃和炔烃的电化学还原偶联反应[J]. 有机化学, 2023, 43(4): 1559-1565. |
| [15] | 梁志鹏, 叶浩, 张海滨, 姜国民, 吴新星. 环丁酮类腙参与的偕二氟环丙烷开环胺化反应[J]. 有机化学, 2023, 43(4): 1483-1491. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||