有机化学 ›› 2022, Vol. 42 ›› Issue (7): 2140-2154.DOI: 10.6023/cjoc202202021 上一篇 下一篇
研究论文
收稿日期:2022-02-18
修回日期:2022-03-23
发布日期:2022-08-09
通讯作者:
姚辉, 黄年玉
基金资助:
Min Hua, Yangxing Sun, Xueqing Zhang, Hui Yao( ), Nianyu Huang(
), Nianyu Huang( )
)
Received:2022-02-18
Revised:2022-03-23
Published:2022-08-09
Contact:
Hui Yao, Nianyu Huang
Supported by:文章分享
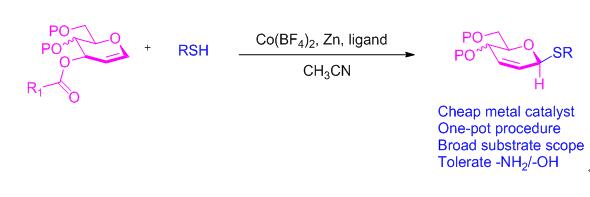
报道了以葡萄烯糖-3-吡啶酸酯和硫酚/硫醇为原料, Co(BF4)2催化下立体选择性合成β-D-葡萄烯糖硫苷的方法. 目标化合物的化学结构经过了核磁共振、高分辨质谱和单晶X射线衍射的确证. 该反应对各类硫醇和硫酚具有良好的官能团兼容性, 能耐受含酯基、酚/醇羟基、氨基和乙酰氨基等活性硫亲核试剂, 还可用于硫糖肽的高效合成, 为硫糖苷的快速制备提供了新方案.
华敏, 孙阳星, 张雪晴, 姚辉, 黄年玉. 廉价钴催化下β-D-葡萄烯糖硫苷的立体选择性合成[J]. 有机化学, 2022, 42(7): 2140-2154.
Min Hua, Yangxing Sun, Xueqing Zhang, Hui Yao, Nianyu Huang. Convenient Cobalt-Catalyzed Stereoselective Synthesis of β-D-Thioglucosides[J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2140-2154.
| Entrya | Donor | R-group | T/℃ | Yieldb/% | β∶αc |
|---|---|---|---|---|---|
| 1 | 1a | | 120d | — | — |
| 2 | 1b | | 80 | 55 | 8∶1 |
| 3 | 1c | | 80 | 50 | 6∶1 |
| 4 | 1d | | 80 | 40 | 3∶1 |
| 5 | 1e | | 80 | 57 | 7∶1 |
| 6 | 1f | | 80 | 40 | 4∶1 |
| Entrya | Donor | R-group | T/℃ | Yieldb/% | β∶αc |
|---|---|---|---|---|---|
| 1 | 1a | | 120d | — | — |
| 2 | 1b | | 80 | 55 | 8∶1 |
| 3 | 1c | | 80 | 50 | 6∶1 |
| 4 | 1d | | 80 | 40 | 3∶1 |
| 5 | 1e | | 80 | 57 | 7∶1 |
| 6 | 1f | | 80 | 40 | 4∶1 |
| Entrya | [Co] | L | Solvent | T/℃ | Yieldb/% | β∶αc |
|---|---|---|---|---|---|---|
| 1 | Co(acac)3 | CataCXium A | CH3CN | 60 | 25 | 7∶1 |
| 2 | Co(acac)3 | CataCXium A | CH3CN | 100 | 59 | 5∶1 |
| 3 | CoBr2 | CataCXium A | CH3CN | 80 | 20 | 1∶3 |
| 4 | CoCl2 | CataCXium A | CH3CN | 80 | 32 | 1∶2 |
| 5 | Co(PPh3)2Cl2 | CataCXium A | CH3CN | 80 | 35 | 4∶1 |
| 6 | Co(acac)2 | CataCXium A | CH3CN | 80 | 50 | 6∶1 |
| 7 | Co(BF4)2 | CataCXium A | CH3CN | 80 | 65 | 8∶1 |
| 8 | Co(BF4)2 | Xantphos | CH3CN | 80 | 45 | 1∶1 |
| 9 | Co(BF4)2 | DPPE | CH3CN | 80 | 66 | 6∶1 |
| 10 | Co(BF4)2 | (R)-(–)-DTBM-SEGPHOS | CH3CN | 80 | 53 | 7∶1 |
| Entrya | [Co] | L | Solvent | T/℃ | Yieldb/% | β∶αc |
| 11 | Co(BF4)2 | Ph3P | CH3CN | 80 | 40 | 4∶1 |
| 12 | Co(BF4)2 | (S,S)-Chiraphos | CH3CN | 80 | 60 | 8∶1 |
| 13 | Co(BF4)2 | DPPF | CH3CN | 80 | 71% | 7:1 |
| 14 | Co(BF4)2 | (R)-(+)-BINAP | CH3CN | 80 | 80% | 10:1 |
| 15 | Co(BF4)2 | (S)-(–)-BINAP | CH3CN | 80 | 50% | 2:1 |
| 16 | Co(BF4)2 | (R)-(+)-BINAP | DMF | 80 | - | - |
| 17 | Co(BF4)2 | (R)-(+)-BINAP | THF | 80 | 47% | 5:1 |
| Entrya | [Co] | L | Solvent | T/℃ | Yieldb/% | β∶αc |
|---|---|---|---|---|---|---|
| 1 | Co(acac)3 | CataCXium A | CH3CN | 60 | 25 | 7∶1 |
| 2 | Co(acac)3 | CataCXium A | CH3CN | 100 | 59 | 5∶1 |
| 3 | CoBr2 | CataCXium A | CH3CN | 80 | 20 | 1∶3 |
| 4 | CoCl2 | CataCXium A | CH3CN | 80 | 32 | 1∶2 |
| 5 | Co(PPh3)2Cl2 | CataCXium A | CH3CN | 80 | 35 | 4∶1 |
| 6 | Co(acac)2 | CataCXium A | CH3CN | 80 | 50 | 6∶1 |
| 7 | Co(BF4)2 | CataCXium A | CH3CN | 80 | 65 | 8∶1 |
| 8 | Co(BF4)2 | Xantphos | CH3CN | 80 | 45 | 1∶1 |
| 9 | Co(BF4)2 | DPPE | CH3CN | 80 | 66 | 6∶1 |
| 10 | Co(BF4)2 | (R)-(–)-DTBM-SEGPHOS | CH3CN | 80 | 53 | 7∶1 |
| Entrya | [Co] | L | Solvent | T/℃ | Yieldb/% | β∶αc |
| 11 | Co(BF4)2 | Ph3P | CH3CN | 80 | 40 | 4∶1 |
| 12 | Co(BF4)2 | (S,S)-Chiraphos | CH3CN | 80 | 60 | 8∶1 |
| 13 | Co(BF4)2 | DPPF | CH3CN | 80 | 71% | 7:1 |
| 14 | Co(BF4)2 | (R)-(+)-BINAP | CH3CN | 80 | 80% | 10:1 |
| 15 | Co(BF4)2 | (S)-(–)-BINAP | CH3CN | 80 | 50% | 2:1 |
| 16 | Co(BF4)2 | (R)-(+)-BINAP | DMF | 80 | - | - |
| 17 | Co(BF4)2 | (R)-(+)-BINAP | THF | 80 | 47% | 5:1 |
| [1] |
(a) Turganbay, S.; Aidarova, S. B.; Bekturganova, N. E.; Li, C. S.; Musabekov, K. B.; Kumargalieva, S. S.; Toshtay, K. Eurasian Chem.-Technol. J. 2012, 14, 313.?
doi: 10.18321/ectj128 |
|
(b) Jacob, C. Nat. Prod. Rep. 2006, 23, 851.
doi: 10.1039/b609523m |
|
|
(c) Devendar, P.; Yang, G. F. Top. Curr. Chem. 2017, 375, 82.
|
|
|
(d) Crockett, M. P.; Evans, A. M.; Worthington, M. J.; Albuquerque, I. S.; Slattery, A. D.; Gibson, C. T.; Campbell, J. A.; Lewis, D. A.; Bernardes, G. J.; Chalker, J. M. Angew. Chem., Int. Ed. 2016, 55, 1714.
doi: 10.1002/anie.201508708 |
|
|
(e) Wang, M.; Wang, C. H.; Jiang, X. F. Chin. J. Org. Chem. 2019, 39, 2139. (in Chinese)
doi: 10.6023/cjoc201903069 |
|
|
( 王明, 王翠红, 姜雪峰, 有机化学, 2019, 39, 2139.)
doi: 10.6023/cjoc201903069 |
|
| [2] |
(a) Driguez, H. ChemBioChem 2001, 2, 311.
pmid: 11828459 |
|
(b) Agrawal, S.; Wozniak, M.; Luc, M.; Walaszek, K.; Pielka, E.; Szeja, W.; Gamian, A.; Ziolkowski, P. Oncotarget 2017, 8, 114173.
doi: 10.18632/oncotarget.23170 pmid: 11828459 |
|
|
(c) Zhu, X.; Stolz, F.; Schmidt, R. R. J. Org. Chem. 2004, 69, 7367.
doi: 10.1021/jo049077m pmid: 11828459 |
|
| [3] |
(a) Witczak, Z. J. Curr. Med. Chem. 1999, 6, 165.
pmid: 10189230 |
|
(b) Witczak, Z. J.; Culhane, J. M. Appl. Microbiol. Biotechnol. 2005, 69, 237.
doi: 10.1007/s00253-005-0156-x pmid: 10189230 |
|
| [4] |
(a) Wang, H.; Zhu, X. Org. Biomol. Chem. 2014, 12, 7119.
doi: 10.1039/C4OB01094A pmid: 19093879 |
|
(b) Zeng, X.; Smith, R.; Zhu, X. J. Org. Chem. 2013, 78, 4165.
doi: 10.1021/jo400274s pmid: 19093879 |
|
|
(c) Gamblin, D. P.; Scanlan, E. M.; Davis, B. G. Chem. Rev. 2009, 109, 131.
doi: 10.1021/cr078291i pmid: 19093879 |
|
| [5] |
(a) Wu, B.; Yang, X.; Yan, M. J. Med. Chem. 2019, 62, 7751.
doi: 10.1021/acs.jmedchem.9b00550 pmid: 23232934 |
|
(b) Tota, A.; Carlucci, C.; Pisano, L.; Cutolo, G.; Clarkson, G. J.; Romanazzi, G.; Luisi, R. Org. Biomol. Chem. 2020, 18, 3893.
doi: 10.1039/D0OB00647E pmid: 23232934 |
|
|
(c) Hutton, M. L.; Pehlivanoglu, H.; Vidor, C. J.; James, M. L.; Thomson, M. J.; Lyras, D. J. Antimicrob. Chemother. 2020, 75, 409.
pmid: 23232934 |
|
|
(d) Umemura, E.; Wakiyama, Y.; Kumura, K.; Ueda, K.; Masaki, S.; Watanabe, T.; Yamamoto, M.; Hirai, Y.; Ajito, K. S. J. Antibiot. 2013, 66, 195.
doi: 10.1038/ja.2012.107 pmid: 23232934 |
|
|
(e) Del-Rosso, J. Q.; Schmidt, N. F. Cutis 2010, 85, 15.
pmid: 23232934 |
|
| [6] |
(a) Yadav, J. S.; Reddy, B. S.; Bhasker, E. V.; Raghavendra, S.; Narsaiah, A. V. Tetrahedron Lett. 2017, 48, 677.
doi: 10.1016/j.tetlet.2006.11.103 |
|
(b) Li, X.; Zhu, J. Eur. J. Org. Chem. 2016, 2016, 4724.
doi: 10.1002/ejoc.201600484 |
|
|
(c) Liu, Q. F.; Zhang, G. S. Chin. J. Org. Chem. 2009, 12, 1890. (in Chinese)
|
|
|
刘青峰, 张贵生, 有机化学, 2009, 12, 1890.)
|
|
| [7] |
(a) Yadav, J. S.; Reddy, B. V. S.; Geetha, V. Synth. Commun. 2003, 33, 717.
doi: 10.1081/SCC-120016313 |
|
(b) Tian, Q.; Zhu, X. M.; Yang, J. S. Synth. Commun. 2007, 37, 691.
doi: 10.1080/00397910601131353 |
|
|
(c) Babu, J.; Khare, A.; Vankar, Y. Molecules 2005, 10, 884.
doi: 10.3390/10080884 |
|
| [8] |
(a) Liu, Y.; Song, T.; Meng, W.; Xu, Y.; Wang, P. G.; Zhao, W. Tetrahedron Lett. 2016, 57, 2758.
doi: 10.1016/j.tetlet.2016.05.026 |
|
(b) Xiang, S.; He, J.; Tan, Y. J.; Liu, X. W. Angew. Chem., Int. Ed. 2015, 54, 604.
|
|
|
(c) Liu, Y.; Jiao, Y.; Luo, H.; Huang, N.; Lai, M.; Zou, K.; Yao, H. ACS Catal. 2021, 11, 5287.
doi: 10.1021/acscatal.1c00225 |
|
| [9] |
(a) McKay, M. J.; Nguyen, H. M. ACS Catal. 2012, 2, 1563.
doi: 10.1021/cs3002513 |
|
(b) Liao, H.; Ma, J.; Yao, H.; Liu, X. W. Org. Biomol. Chem. 2018, 16, 1791.
doi: 10.1039/C8OB00032H |
|
| [10] |
(a) Liu, X.; Lin, Y.; Liu, A.; Sun, Q.; Sun, H.; Xu, P.; Li, W. Chin. J. Chem. 2022, 40, 443.
doi: 10.1002/cjoc.202100865 pmid: 18366046 |
|
(b) Dziuba, K.; Lubańska, M.; Pietrusiewicz, K. M. Synthesis 2020, 52, 909.
doi: 10.1055/s-0039-1691531 pmid: 18366046 |
|
|
(c) Bedford, R. B.; Cazin, C. S. Chem. Commun. 2001, 17, 1540.
pmid: 18366046 |
|
|
(d) Fleckenstein, C. A.; Plenio, H. Chem.-Eur. J. 2008, 14, 4267.
doi: 10.1002/chem.200701877 pmid: 18366046 |
|
| [11] |
(a) Gómez, A. M.; Lobo, F.; Uriel, C.; López, J. C. Eur. J. Org. Chem. 2013, 2013, 7221.
doi: 10.1002/ejoc.201300798 pmid: 26732258 |
|
(b) Gómez, A. M.; Miranda, S.; López, J. C. Carbohydr. Chem. 2016, 42, 210.
pmid: 26732258 |
|
|
(c) Minbiole, E. C.; Minbiole, K. P. J. Antibiot. 2016, 69, 213.
doi: 10.1038/ja.2015.136 pmid: 26732258 |
|
| [12] |
(a) Xiang, S.; Lu, Z.; He, J.; Zeng, J.; Liu, X. W. Chem.-Eur. J. 2013, 19, 14047.
doi: 10.1002/chem.201303241 pmid: 25730324 |
|
(b) Ji, L.; Xiang, S. H.; Leng, W. L.; Liu, X. W. Org. Lett. 2015, 17, 1357.
doi: 10.1021/ol5037437 pmid: 25730324 |
|
| [13] |
Kim, H.; Men, H.; Lee, C. J. Am. Chem. Soc. 2004, 126, 1336.
doi: 10.1021/ja039746y |
| [14] |
(a) Sun, M.; Chen, J. F.; Chen, S.; Li, C. Org. Lett. 2019, 21, 1278.
doi: 10.1021/acs.orglett.8b04030 pmid: 31274311 |
|
(b) Ghorai, S.; Chirke, S. S.; Xu, W. B.; Chen, J. F.; Li, C. J. Am. Chem. Soc. 2019, 141, 11430.
doi: 10.1021/jacs.9b06035 pmid: 31274311 |
| [1] | 冯康博, 陈炯, 古双喜, 王海峰, 陈芬儿. 全连续流反应技术在药物合成中的新进展(2019~2022)[J]. 有机化学, 2024, 44(2): 378-397. |
| [2] | 李鹏辉, 谢青洋, 万福贤, 张元红, 姜林. 含环丙基的新型取代嘧啶-5-甲酰胺的合成及杀菌活性研究[J]. 有机化学, 2024, 44(2): 650-656. |
| [3] | 邹发凯, 王能中, 姚辉, 王慧, 刘明国, 黄年玉. 1β-/3R-芳基硫代糖的区域与立体选择性合成[J]. 有机化学, 2024, 44(2): 593-604. |
| [4] | 李路瑶, 贺忠文, 张振国, 贾振华, 罗德平. 三芳基碳正离子在有机合成中的应用[J]. 有机化学, 2024, 44(2): 421-437. |
| [5] | 梅青刚, 李清寒. 可见光促进C(3)(杂)芳硫基吲哚化合物的合成研究进展[J]. 有机化学, 2024, 44(2): 398-408. |
| [6] | 杨维清, 葛宴兵, 陈元元, 刘萍, 付海燕, 马梦林. 1,8-萘酰亚胺衍生物的设计、合成及其对半胱氨酸的识别研究[J]. 有机化学, 2024, 44(1): 180-194. |
| [7] | 赵茜帆, 陈永正, 张世明. 碳基非金属催化剂在有机合成领域的应用及机理研究[J]. 有机化学, 2024, 44(1): 137-147. |
| [8] | 陈珊, 陈志林, 胡琼, 蒙艳双, 黄悦, 陶萍芳, 卢丽如, 黄国保. 含双硫脲基团分子钳在非极性溶剂中识别中性分子[J]. 有机化学, 2024, 44(1): 277-281. |
| [9] | 王化坤, 任晓龙, 宣宜宁. 卤盐催化的α,β-环氧羧酸酯与异氰酸酯[3+2]环加成反应研究[J]. 有机化学, 2024, 44(1): 251-258. |
| [10] | 金玉坤, 任保轶, 梁福顺. 可见光介导的三氟甲基的选择性C-F键断裂及其在偕二氟类化合物合成中的应用[J]. 有机化学, 2024, 44(1): 85-110. |
| [11] | 马翠云, 罗海澜, 张福华, 郭丹, 陈树兴, 王飞. 3-Pyrrolyl BODIPY的绿色生物合成、光物理性质及应用研究[J]. 有机化学, 2024, 44(1): 216-223. |
| [12] | 王博珍, 张婕, 粘春惠, 金茗茗, 孔苗苗, 李物兰, 何文斐, 吴建章. 含有3,4-二氯苯基的酰胺类化合物的合成及抗肿瘤活性研究[J]. 有机化学, 2024, 44(1): 232-241. |
| [13] | 于士航, 刘嘉威, 安碧玉, 边庆花, 王敏, 钟江春. 黑腹尼虎天牛接触性信息素的不对称合成[J]. 有机化学, 2024, 44(1): 301-308. |
| [14] | 李阳, 袁锦鼎, 赵頔. 低共熔溶剂1,3-二甲基脲/L-(+)-酒石酸中(E)-2-苯乙烯基喹啉-3-羧酸类衍生物的绿色合成[J]. 有机化学, 2023, 43(9): 3268-3276. |
| [15] | 岁丹丹, 岑南楠, 龚若蕖, 陈阳, 陈文博. 无支持电解质条件下连续流电化学合成三氟甲基化氧化吲哚[J]. 有机化学, 2023, 43(9): 3239-3245. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||