有机化学 ›› 2022, Vol. 42 ›› Issue (11): 3843-3852.DOI: 10.6023/cjoc202205008 上一篇 下一篇
研究论文
应安国a, 白林盛a, 侯海亮b, 许松林b, 鲁小彤a, 王丽敏a,*( )
)
收稿日期:2022-05-05
修回日期:2022-06-10
发布日期:2022-07-05
通讯作者:
王丽敏
基金资助:
Anguo Yinga, Linsheng Baia, Hailiang Houb, Songlin Xub, Xiaotong Lua, Limin Wanga( )
)
Received:2022-05-05
Revised:2022-06-10
Published:2022-07-05
Contact:
Limin Wang
Supported by:文章分享

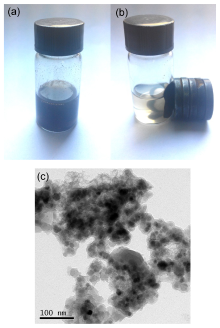
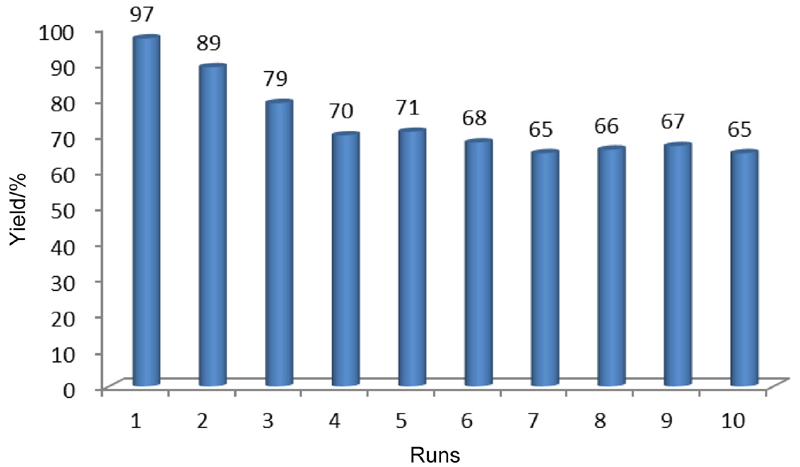
利用化学共沉淀法制备了超顺磁性Fe3O4纳米颗粒, 然后在碱性环境中水解正硅酸四乙酯得到二氧化硅包覆的核壳结构(MNPs), 进一步负载AlCl3得到非均相催化剂AlCl3@MNPs. 采用X射线电子能谱、X射线衍射、透射电镜、扫描电镜及磁滞回线测试等手段对AlCl3@MNPs的物化性能和结构进行表征, 表明氯化铝通过与载体表面弱配位键的形成成功负载到磁性纳米粒上. 将得到的超顺磁性纳米粒负载的氯化铝(AlCl3@MNPs)应用到硫杂Michael加成串联反应中. 研究发现, 该催化剂能有效促进胺、二硫化碳和α,β-不饱和羰基化合物加成反应, 产品收率为59%~99%. 催化剂具备的超顺磁性纳米颗粒的大表面积、反应时的良好分散性和AlCl3与载体表面羟基的配位作用保证了其高催化活性. 另外, 由于AlCl3@MNPs的超顺磁性, 在外加磁场存在条件下可以简便回收, 并重复使用10次, 其催化效果未见大幅下降, 明显优于未负载AlCl3母体, 这显示了其良好的工业化应用前景.
应安国, 白林盛, 侯海亮, 许松林, 鲁小彤, 王丽敏. AlCl3@MNPs催化硫杂Michael加成串联反应研究[J]. 有机化学, 2022, 42(11): 3843-3852.
Anguo Ying, Linsheng Bai, Hailiang Hou, Songlin Xu, Xiaotong Lu, Limin Wang. Research on Thia-Michael Addition Tandem Reactions Catalyzed by AlCl3@MNPs[J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3843-3852.
| Entry | Catalyst (g) | Solvent | Time/h | Yieldb/% |
|---|---|---|---|---|
| 1 | AlCl3@MNPs (0.20) | Ethanol | 5 | 97 |
| 2 | AlCl3 (0.04) | Ethanol | 5 | 95 |
| 3 | FeCl3 (0.04) | Ethanol | 5 | 53 |
| 4 | ZnCl2 (0.04) | Ethanol | 5 | 38 |
| 3 | MNPs (0.16) | Ethanol | 10 | Trace |
| 4 | — | Ethanol | 14 | Trace |
| 5 | AlCl3@MNPs (0.20) | Methanol | 5 | 93 |
| 6 | AlCl3@MNPs (0.2) | CH2Cl2 | 6 | 82 |
| 7 | AlCl3@MNPs (0.2) | Toluene | 6 | 84 |
| 8 | AlCl3@MNPs (0.01) | Ethanol | 8 | 30 |
| 9 | AlCl3@MNPs (0.05) | Ethanol | 6 | 58 |
| 10 | AlCl3@MNPs (0.10) | Ethanol | 5 | 76 |
| 11 | AlCl3@MNPs (0.25) | Ethanol | 5 | 96 |
| Entry | Catalyst (g) | Solvent | Time/h | Yieldb/% |
|---|---|---|---|---|
| 1 | AlCl3@MNPs (0.20) | Ethanol | 5 | 97 |
| 2 | AlCl3 (0.04) | Ethanol | 5 | 95 |
| 3 | FeCl3 (0.04) | Ethanol | 5 | 53 |
| 4 | ZnCl2 (0.04) | Ethanol | 5 | 38 |
| 3 | MNPs (0.16) | Ethanol | 10 | Trace |
| 4 | — | Ethanol | 14 | Trace |
| 5 | AlCl3@MNPs (0.20) | Methanol | 5 | 93 |
| 6 | AlCl3@MNPs (0.2) | CH2Cl2 | 6 | 82 |
| 7 | AlCl3@MNPs (0.2) | Toluene | 6 | 84 |
| 8 | AlCl3@MNPs (0.01) | Ethanol | 8 | 30 |
| 9 | AlCl3@MNPs (0.05) | Ethanol | 6 | 58 |
| 10 | AlCl3@MNPs (0.10) | Ethanol | 5 | 76 |
| 11 | AlCl3@MNPs (0.25) | Ethanol | 5 | 96 |
| Entry | R1 | R2 | Time/h | 8 | Yieldb/% |
|---|---|---|---|---|---|
| 1 | 4-Me-piperidyl | NH2 | 5 | 8a | 97 |
| 2 | 4-Me-piperidyl | N(CH3)2 | 6 | 8b | 90 |
| 3 | 4-Me-piperidyl | NHCH(CH3)2 | 6 | 8c | 92 |
| 4 | 4-Me-piperidyl | Morpholinyl | 6 | 8d | 93 |
| 5 | 4-Me-piperidyl | NHCH2OH | 6 | 8e | 95 |
| 6 | 4-Me-piperidyl | NHC(CH3)3 | 7 | 8f | 68 |
| 7 | 4-Me-piperidyl | NHC(CH3)2- CH2COCH3 | 8 | 8g | 59 |
| 8 | 4-Me-piperidyl | OCH3 | 5 | 8h | 99 |
| 9 | Piperidyl | NH2 | 5 | 8i | 86 |
| 10 | Piperidyl | NHCH2OH | 6 | 8j | 90 |
| 11 | Piperidyl | Morpholinyl | 6 | 8k | 86 |
| 12 | Piperidyl | NHCH(CH3)2 | 6 | 8l | 87 |
| 13 | Piperidyl | N(CH3)2 | 5 | 8m | 87 |
| 14 | Morpholinyl | Morpholinyl | 6 | 8n | 80 |
| 15 | Morpholinyl | N(CH3)2 | 6 | 8o | 90 |
| 16 | Morpholinyl | OCH3 | 5 | 8p | 86 |
| 17 | Pyrrolidyl | NHCH(CH3)2 | 6 | 8q | 95 |
| 18 | Pyrrolidyl | NHCH2OH | 6 | 8r | 95 |
| 19 | Pyrrolidyl | Morpholinyl | 6 | 8s | 85 |
| 20 | Pyrrolidyl | N(CH3)2 | 6 | 8t | 84 |
| Entry | R1 | R2 | Time/h | 8 | Yieldb/% |
|---|---|---|---|---|---|
| 1 | 4-Me-piperidyl | NH2 | 5 | 8a | 97 |
| 2 | 4-Me-piperidyl | N(CH3)2 | 6 | 8b | 90 |
| 3 | 4-Me-piperidyl | NHCH(CH3)2 | 6 | 8c | 92 |
| 4 | 4-Me-piperidyl | Morpholinyl | 6 | 8d | 93 |
| 5 | 4-Me-piperidyl | NHCH2OH | 6 | 8e | 95 |
| 6 | 4-Me-piperidyl | NHC(CH3)3 | 7 | 8f | 68 |
| 7 | 4-Me-piperidyl | NHC(CH3)2- CH2COCH3 | 8 | 8g | 59 |
| 8 | 4-Me-piperidyl | OCH3 | 5 | 8h | 99 |
| 9 | Piperidyl | NH2 | 5 | 8i | 86 |
| 10 | Piperidyl | NHCH2OH | 6 | 8j | 90 |
| 11 | Piperidyl | Morpholinyl | 6 | 8k | 86 |
| 12 | Piperidyl | NHCH(CH3)2 | 6 | 8l | 87 |
| 13 | Piperidyl | N(CH3)2 | 5 | 8m | 87 |
| 14 | Morpholinyl | Morpholinyl | 6 | 8n | 80 |
| 15 | Morpholinyl | N(CH3)2 | 6 | 8o | 90 |
| 16 | Morpholinyl | OCH3 | 5 | 8p | 86 |
| 17 | Pyrrolidyl | NHCH(CH3)2 | 6 | 8q | 95 |
| 18 | Pyrrolidyl | NHCH2OH | 6 | 8r | 95 |
| 19 | Pyrrolidyl | Morpholinyl | 6 | 8s | 85 |
| 20 | Pyrrolidyl | N(CH3)2 | 6 | 8t | 84 |

| [1] |
Dickinsonl, W. B.; Vaupotic, M. P. US 4039550, 1977.
|
| [2] |
Bergman, R. W.; Smith, H. A. US 4618461, 1986.
|
| [3] |
Mashhadizadeh, M. H.; Talemi, R, P.; Shockravi, A.; Kamali, M. Anal. Methods 2012, 4, 742.
doi: 10.1039/c2ay05559g |
| [4] |
Meng, X. M.; Liu, L.; Hu, H. Y.; Zhu, M. H.; Wang, M. X.; Shi, J.; Guo, Q. X. Tetrahedron Lett. 2006, 47, 7961.
doi: 10.1016/j.tetlet.2006.08.127 |
| [5] |
Kondoh, A.; Hirozane, T.; Terada, M. Chem. Eur. J. 2022, e202201240.
|
| [6] |
Xie, Q. X.; Liu, L. X.; Zhu, Z. H.; Yu, C. B.; Zhou, Y. G. J. Org. Chem. 2022, 87, 7521.
doi: 10.1021/acs.joc.2c00418 |
| [7] |
Khan, A. T.; Ali, S.; Dar, A. A.; Lal, M. Tetrahedron Lett. 2011, 52, 5157.
doi: 10.1016/j.tetlet.2011.07.113 |
| [8] |
Saidi, M. R.; Azizi, N.; Akbari, E.; Ebrahimi, F. J. Mol. Catal. A: Chem. 2008, 292, 44.
doi: 10.1016/j.molcata.2008.06.003 |
| [9] |
Quadrado, R. F. N.; Macagnan, K. L.; Moreira, A. S.; Rajardo, A. R. Int. J. Biol. Macromol. 2021, 193, 1032.
doi: 10.1016/j.ijbiomac.2021.11.075 pmid: 34800516 |
| [10] |
Kshiar, B.; Shangpliang, Q. R.; Myrboh, B. Synth. Commun. 2018, 48, 1816.
doi: 10.1080/00397911.2018.1468467 |
| [11] |
Xu, S. B.; Li, C. J.; Jia, X. S.; Li, J. J. Org. Chem. 2014, 79, 11161.
doi: 10.1021/jo502209f |
| [12] |
Yang, H.; Ning, Z.; Wang, S.; Li, J.; Wang, Z.; Wang, W. L.; Xu, X. M. Tetrahedron Lett. 2021, 74, 153174.
doi: 10.1016/j.tetlet.2021.153174 |
| [13] |
Karmakar, B.; Banerji, J. Tetrahedron Lett. 2011, 52, 6584.
doi: 10.1016/j.tetlet.2011.09.131 |
| [14] |
Azizi, N.; Khajeh, M.; Hasani, M.; Dezfooli, S. Tetrahedron Lett. 2013, 54, 5407.
|
| [15] |
Ying, A.; Li, Z.; Yang, J.; Liu, S.; Xu, S.; Yan, H.; Wu, C. J. Org. Chem. 2014, 79, 6510.
doi: 10.1021/jo500937a |
| [16] |
Gupta, R.; Yadav, M.; Gaur, R.; Arora, G.; Rana, P.; Yadav, P.; Adholeya, A.; Sharma, R. K. ACS Omega 2019, 4, 21529.
doi: 10.1021/acsomega.9b03237 |
| [17] |
Gawande, M. B.; Branco, P. S.; Varma, R. S. Chem. Soc. Rev. 2013, 42, 3371.
doi: 10.1039/c3cs35480f pmid: 23420127 |
| [18] |
Sharma, R. K.; Dutta, S.; Sharma, S.; Zboril, R.; Varma, R. S.; Gawande, M. B. Green Chem. 2016, 18, 3184.
doi: 10.1039/C6GC00864J |
| [19] |
Saxena, M.; Saxena, R. Mater. Chem. Phys. 2022, 276, 125437.
doi: 10.1016/j.matchemphys.2021.125437 |
| [20] |
Haqjow, H.; Raoufi, F. Res. Chem. Intermed. 2021, 47, 4113.
doi: 10.1007/s11164-021-04522-7 |
| [21] |
Ying, A.; Liu, S.; Li, Z.; Chen, G.; Yang, J.; Yan, H.; Xu, S. Adv. Synth. Catal. 2016, 358, 2116.
doi: 10.1002/adsc.201600145 |
| [22] |
Wang, A.; Sudarsanam, P.; Xu, Y.; Zhang, H.; Li, H.; Yang, S. Green Chem. 2020, 22, 2977.
doi: 10.1039/D0GC00924E |
| [23] |
Lu, X.; Li, S.; Wang, L.; Huang, S.; Liu, Z.; Liu, Y.; Ying, A. Fuel 2022, 310, 122318.
doi: 10.1016/j.fuel.2021.122318 |
| [24] |
Chen, Z.; Yao, J.; Ma, B.; Liu, B.; Kim, J.; Li, H.; Zhu, X.; Zhao, C.; Amde, M. Chemosphere 2022, 291, 132727.
doi: 10.1016/j.chemosphere.2021.132727 |
| [25] |
Ying, Q.; Chen, H.; Shao, P.; Zhou, X.; He, X.; Ye, J.; Zhang, S.; Chen, J.; Wang, L. J. CO2 Util. 2021, 49, 101565.
|
| [26] |
Gawande, M. B.; Branco, P. S.; Varma, R. S. Chem. Soc. Rev. 2013, 42, 3371.
doi: 10.1039/c3cs35480f pmid: 23420127 |
| [27] |
McGuire, G. E.; Schweitzer, G. K.; Thomas, T. A. Inorg. Chem. 1973, 12, 2450.
doi: 10.1021/ic50128a045 |
| [28] |
Deng, R. J.; You, K. Y.; Yi, L.; Zhao, F. F.; Jian, J.; Chen, Z. P.; Liu, P.; Ai, Q. H.; Luo, H. A. Ind. Eng. Chem. Res. 2018, 57, 12993.
doi: 10.1021/acs.iecr.8b02786 |
| [29] |
Azizi, N.; Aryanasab, F.; Torkiyan, L.; Ziyaei, A.; Saidi, M. R. J. Org. Chem. 2006, 71, 3634.
doi: 10.1021/jo060048g |
| [1] | 黄净, 杨毅华, 张占辉, 刘守信. 酰胺键的绿色高效构建方法与技术进展[J]. 有机化学, 2024, 44(2): 409-420. |
| [2] | 岁丹丹, 岑南楠, 龚若蕖, 陈阳, 陈文博. 无支持电解质条件下连续流电化学合成三氟甲基化氧化吲哚[J]. 有机化学, 2023, 43(9): 3239-3245. |
| [3] | 蒋宜欣, 唐伯孝, 毛海波, 陈雪霞, 俞洋杰, 全翠英, 徐昭阳, 石金慧, 刘益林. 水-聚乙二醇(PEG-200)中烯烃与碘代芳烃绿色可循环无负载偶联反应的研究[J]. 有机化学, 2023, 43(9): 3210-3215. |
| [4] | 卢凯, 屈浩琦, 陈樨, 秋慧, 郑晶, 马猛涛. 无催化剂、无溶剂条件下炔烃和烯烃与儿茶酚硼烷的硼氢化反应[J]. 有机化学, 2023, 43(6): 2197-2205. |
| [5] | 莫百川, 陈春霞, 彭进松. 木质素及其衍生物负载金属催化剂在有机合成中的应用研究进展[J]. 有机化学, 2023, 43(4): 1215-1240. |
| [6] | 窦谦, 汪太民, 房丽晶, 翟宏斌, 程斌. 光诱导铁催化在有机合成中的应用研究进展[J]. 有机化学, 2023, 43(4): 1386-1415. |
| [7] | 李奇阳, 张海燕, 刘文博. 无过渡金属参与的碳硅键构筑方法研究进展[J]. 有机化学, 2023, 43(10): 3470-3490. |
| [8] | 宇世伟, 陈兆华, 陈淇, 林舒婷, 何金萍, 陶冠燊, 汪朝阳. 硫代磺酸酯的合成与应用研究进展[J]. 有机化学, 2022, 42(8): 2322-2330. |
| [9] | 顾清云, 程振凤, 曾小宝. 电化学氧化三氟甲基亚磺酸钠与α-羰基二硫缩烯酮的三氟甲基化反应[J]. 有机化学, 2022, 42(5): 1537-1544. |
| [10] | 郑煜, 钱沈城, 徐鹏程, 郑斌南, 黄申林. 电化学氧化芳基端炔的硫氰化磺化反应[J]. 有机化学, 2022, 42(12): 4275-4281. |
| [11] | 李红霞, 陈棚, 伍智林, 陆雨函, 彭俊梅, 陈锦杨, 何卫民. 电化学促进的五元芳香杂环与硫氰酸铵氧化交叉脱氢偶联反应[J]. 有机化学, 2022, 42(10): 3398-3404. |
| [12] | 张瑶瑶, 周丽洁, 韩彪, 李维双, 李博解, 朱磊. 壳聚糖负载铜催化剂在有机反应中的应用研究进展[J]. 有机化学, 2022, 42(1): 33-53. |
| [13] | 蒙泽银, 冯承涛, 徐坤. 基于电化学方法构建碳-氮键的最新研究进展[J]. 有机化学, 2021, 41(7): 2535-2570. |
| [14] | 赵志恒, 李鸣, 周娅琴, 何永辉, 张丽珠, 李干鹏, 谷利军. 电化学脱氢[3+2]环化反应合成取代的1,2,4-三氮唑衍生物[J]. 有机化学, 2021, 41(6): 2476-2484. |
| [15] | 吴媚, 于玲, 侯慧青, 陈厚铮, 庄庆龙, 周孙英, 林小燕. 水相中电化学促进铜催化苯甲醇氧化合成喹唑啉酮[J]. 有机化学, 2021, 41(6): 2326-2334. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||