有机化学 ›› 2023, Vol. 43 ›› Issue (2): 686-696.DOI: 10.6023/cjoc202206030 上一篇 下一篇
研究论文
李猛†, 夏东国†, 王云霄, 程祥, 巩杰秀, 陈耀, 吕献海*( )
)
收稿日期:2022-06-18
修回日期:2022-09-04
发布日期:2022-10-24
作者简介:基金资助:
Meng Li†, Dongguo Xia†, Yunxiao Wang, Xiang Cheng, Jiexiu Gong, Yao Chen, Xianhai Lü( )
)
Received:2022-06-18
Revised:2022-09-04
Published:2022-10-24
Contact:
*E-mail: About author:Supported by:文章分享
植物病原真菌严重威胁作物安全, 迫切需要开发新型高效低毒的杀菌剂. 因此, 设计、合成了一系列新型苯甲酸噻唑酯衍生物. 体外抗真菌活性筛选结果表明, 化合物3、5和8对灰霉病菌和核盘病菌均表现出良好的抗真菌活性. 其中化合物8对灰霉病菌和核盘病菌的EC50值分别为13.82和3.66 mg/L. 初步的生理生化实验结果表明化合物8对菌丝细胞膜造成一定程度的损伤.
李猛, 夏东国, 王云霄, 程祥, 巩杰秀, 陈耀, 吕献海. 苯甲酸噻唑酯类衍生物的设计、合成及抗真菌活性评价[J]. 有机化学, 2023, 43(2): 686-696.
Meng Li, Dongguo Xia, Yunxiao Wang, Xiang Cheng, Jiexiu Gong, Yao Chen, Xianhai Lü. Design, Synthesis and Antifungal Bioactivity Evaluation of Thiazole Benzoate Derivatives[J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 686-696.
| Compd. | R1 | R2 | Inhibition ratea/% | ||||
|---|---|---|---|---|---|---|---|
| B. cinereab | V. malic | S. sclerotiorumd | |||||
| 1 | Phenyl | H | 15.4±0.7 | 25.8±1.2 | 30.2±1.3 | ||
| 2 | 1-Naphthyl | H | 45.6±0.9 | 16.9±1.4 | 10.5±1.8 | ||
| 3 | Isopropyl | H | 72.3±1.5 | 72.6±0.9 | 83.2±1.1 | ||
| 4 | 2-Cl-Phenyl | H | 22.6±1.5 | 41.3±0.8 | 20.1±0.8 | ||
| 5 | 3-Cl-Phenyl | H | 77.4±2.2 | 38.7±1.6 | 56.0±1.4 | ||
| 6 | 4-Cl-Phenyl | H | 32.3±1.1 | 12.9±0.7 | 26.3±2.9 | ||
| 7 | 2-CH3-Phenyl | H | 13.1±2.4 | 21.6±1.2 | 43.5±0.9 | ||
| 8 | 3-CH3-Phenyl | H | 95.3±3.5 | 61.2±1.8 | 94.9±1.6 | ||
| 9 | 4-CH3-Phenyl | H | 23.0±1.5 | 31.5±1.4 | 18.4±0.7 | ||
| 10 | Isopropyl | 2-Cl | 30.5±0.9 | 61.5±0.3 | 20.1±0.3 | ||
| 11 | Isopropyl | 3-Cl | 86.9±2.0 | 53.4±2.5 | 90.9±1.1 | ||
| 12 | Isopropyl | 4-Cl | 83.3±1.3 | 41.9±2.2 | 85.2±0.3 | ||
| 13 | Isopropyl | 2-CH3 | 16.4±0.5 | 63.3±1.0 | 67.6±1.4 | ||
| 14 | Isopropyl | 3-CH3 | 90.6±0.7 | 81.6±2.4 | 88.2±2.1 | ||
| 15 | Isopropyl | 4-CH3 | 44.3±1.9 | 65.3±2.0 | 65.2±1.6 | ||
| 16 | Isopropyl | 2-CF3 | 19.1±0.6 | 61.2±1.3 | 63.8±0.3 | ||
| 17 | Isopropyl | 3-CF3 | 48.3±1.3 | 61.6±0.5 | 33.6±0.6 | ||
| 18 | Isopropyl | 4-CF3 | 30.6±1.7 | 57.5±1.1 | 65.0±1.2 | ||
| 19 | 3-Cl-Phenyl | 2-Cl | 82.2±0.6 | 55.1±1.9 | 56.0±1.8 | ||
| 20 | 3-Cl-Phenyl | 3-Cl | 11.5±0.5 | 30.6±0.7 | 5.1±1.2 | ||
| 21 | 3-Cl-phenyl | 4-Cl | 82.9±2.0 | 51.0±1.5 | 85.3±0.9 | ||
| 22 | 3-Cl-Phenyl | 2-CH3 | 88.2±1.5 | 12.3±0.3 | 95.1±1.4 | ||
| 23 | 3-Cl-Phenyl | 3-CH3 | 16.4±1.9 | 9.8±1.3 | 61.6±0.4 | ||
| 24 | 3-Cl-Phenyl | 4-CH3 | 40.1±0.8 | 8.2±1.4 | 81.6±0.2 | ||
| 25 | 3-Cl-Phenyl | 2-CF3 | 45.3±9.5 | 14.5±0.2 | 56.2±1.3 | ||
| 26 | 3-Cl-Phenyl | 3-CF3 | 35.8±1.2 | 16.2±0.6 | 40.8±1.8 | ||
| 27 | 3-Cl-Phenyl | 4-CF3 | 21.5±0.4 | 65.1±2.2 | 30.8±1.6 | ||
| 28 | 3-CH3-Phenyl | 2-Cl | 84.4±0.6 | 55.6±1.2 | 26.4±1.3 | ||
| 30 | 3-CH3-Phenyl | 4-Cl | 60.9±1.2 | 36.9±0.5 | 40.6±0.7 | ||
| 31 | 3-CH3-Phenyl | 2-CH3 | 57.3±2.0 | 45.4±1.3 | 35.1±0.3 | ||
| 32 | 3-CH3-Phenyl | 3-CH3 | 56.7±0.7 | 26.4±0.5 | 16.7±1.4 | ||
| Compd. | R1 | R2 | Inhibition ratea/% | ||||
| B. cinereab | V. malic | S. sclerotiorumd | |||||
| 33 | 3-CH3-Phenyl | 4-CH3 | 44.3±0.5 | 61.0±2.2 | 19.2±2.1 | ||
| 34 | 3-CH3-Phenyl | 2-CF3 | 58.0±1.5 | 20.3±0.4 | 15.6±1.5 | ||
| 35 | 3-CH3-Phenyl | 3-CF3 | 65.4±1.9 | 9.1±1.5 | 6.4±0.5 | ||
| 36 | 3-CH3-Phenyl | 4-CF3 | 45.6±1.3 | 30.7±1.1 | 46.5±1.8 | ||
| Boscalid | - | - | 100.0 | 95.3 | 100.0 | ||
| Compd. | R1 | R2 | Inhibition ratea/% | ||||
|---|---|---|---|---|---|---|---|
| B. cinereab | V. malic | S. sclerotiorumd | |||||
| 1 | Phenyl | H | 15.4±0.7 | 25.8±1.2 | 30.2±1.3 | ||
| 2 | 1-Naphthyl | H | 45.6±0.9 | 16.9±1.4 | 10.5±1.8 | ||
| 3 | Isopropyl | H | 72.3±1.5 | 72.6±0.9 | 83.2±1.1 | ||
| 4 | 2-Cl-Phenyl | H | 22.6±1.5 | 41.3±0.8 | 20.1±0.8 | ||
| 5 | 3-Cl-Phenyl | H | 77.4±2.2 | 38.7±1.6 | 56.0±1.4 | ||
| 6 | 4-Cl-Phenyl | H | 32.3±1.1 | 12.9±0.7 | 26.3±2.9 | ||
| 7 | 2-CH3-Phenyl | H | 13.1±2.4 | 21.6±1.2 | 43.5±0.9 | ||
| 8 | 3-CH3-Phenyl | H | 95.3±3.5 | 61.2±1.8 | 94.9±1.6 | ||
| 9 | 4-CH3-Phenyl | H | 23.0±1.5 | 31.5±1.4 | 18.4±0.7 | ||
| 10 | Isopropyl | 2-Cl | 30.5±0.9 | 61.5±0.3 | 20.1±0.3 | ||
| 11 | Isopropyl | 3-Cl | 86.9±2.0 | 53.4±2.5 | 90.9±1.1 | ||
| 12 | Isopropyl | 4-Cl | 83.3±1.3 | 41.9±2.2 | 85.2±0.3 | ||
| 13 | Isopropyl | 2-CH3 | 16.4±0.5 | 63.3±1.0 | 67.6±1.4 | ||
| 14 | Isopropyl | 3-CH3 | 90.6±0.7 | 81.6±2.4 | 88.2±2.1 | ||
| 15 | Isopropyl | 4-CH3 | 44.3±1.9 | 65.3±2.0 | 65.2±1.6 | ||
| 16 | Isopropyl | 2-CF3 | 19.1±0.6 | 61.2±1.3 | 63.8±0.3 | ||
| 17 | Isopropyl | 3-CF3 | 48.3±1.3 | 61.6±0.5 | 33.6±0.6 | ||
| 18 | Isopropyl | 4-CF3 | 30.6±1.7 | 57.5±1.1 | 65.0±1.2 | ||
| 19 | 3-Cl-Phenyl | 2-Cl | 82.2±0.6 | 55.1±1.9 | 56.0±1.8 | ||
| 20 | 3-Cl-Phenyl | 3-Cl | 11.5±0.5 | 30.6±0.7 | 5.1±1.2 | ||
| 21 | 3-Cl-phenyl | 4-Cl | 82.9±2.0 | 51.0±1.5 | 85.3±0.9 | ||
| 22 | 3-Cl-Phenyl | 2-CH3 | 88.2±1.5 | 12.3±0.3 | 95.1±1.4 | ||
| 23 | 3-Cl-Phenyl | 3-CH3 | 16.4±1.9 | 9.8±1.3 | 61.6±0.4 | ||
| 24 | 3-Cl-Phenyl | 4-CH3 | 40.1±0.8 | 8.2±1.4 | 81.6±0.2 | ||
| 25 | 3-Cl-Phenyl | 2-CF3 | 45.3±9.5 | 14.5±0.2 | 56.2±1.3 | ||
| 26 | 3-Cl-Phenyl | 3-CF3 | 35.8±1.2 | 16.2±0.6 | 40.8±1.8 | ||
| 27 | 3-Cl-Phenyl | 4-CF3 | 21.5±0.4 | 65.1±2.2 | 30.8±1.6 | ||
| 28 | 3-CH3-Phenyl | 2-Cl | 84.4±0.6 | 55.6±1.2 | 26.4±1.3 | ||
| 30 | 3-CH3-Phenyl | 4-Cl | 60.9±1.2 | 36.9±0.5 | 40.6±0.7 | ||
| 31 | 3-CH3-Phenyl | 2-CH3 | 57.3±2.0 | 45.4±1.3 | 35.1±0.3 | ||
| 32 | 3-CH3-Phenyl | 3-CH3 | 56.7±0.7 | 26.4±0.5 | 16.7±1.4 | ||
| Compd. | R1 | R2 | Inhibition ratea/% | ||||
| B. cinereab | V. malic | S. sclerotiorumd | |||||
| 33 | 3-CH3-Phenyl | 4-CH3 | 44.3±0.5 | 61.0±2.2 | 19.2±2.1 | ||
| 34 | 3-CH3-Phenyl | 2-CF3 | 58.0±1.5 | 20.3±0.4 | 15.6±1.5 | ||
| 35 | 3-CH3-Phenyl | 3-CF3 | 65.4±1.9 | 9.1±1.5 | 6.4±0.5 | ||
| 36 | 3-CH3-Phenyl | 4-CF3 | 45.6±1.3 | 30.7±1.1 | 46.5±1.8 | ||
| Boscalid | - | - | 100.0 | 95.3 | 100.0 | ||
| Fungi | Compd. | EC50/(mg•L-1) | 95% confidence interval | Regression equationa | Rb |
|---|---|---|---|---|---|
| B. cinerea | 3 | 32.61 | 29.13~36.99 | y=5.581x-8.446 | 0.998 |
| 5 | 31.60 | 28.24~35.81 | y=5.550x-8.323 | 0.988 | |
| 8 | 13.82 | 12.27~15.58 | y=4.690x-5.349 | 0.989 | |
| 11 | 21.17 | 18.97~23.72 | y=5.422x-7.188 | 0.989 | |
| 12 | 21.81 | 14.06~40.87 | y=3.120x-4.176 | 0.942 | |
| 14 | 15.31 | 13.56~17.32 | y=4.513x-5.347 | 0.994 | |
| 19 | 21.85 | 19.01~25.43 | y=3.941x-5.010 | 0.986 | |
| 21 | 23.36 | 15.85~40.05 | y=3.498x-4.787 | 0.962 | |
| 22 | 19.25 | 17.11~21.77 | y=4.765x-6.120 | 0.995 | |
| 28 | 23.17 | 20.27~26.85 | y=3.974x-5.420 | 0.980 | |
| Boscalid | 0.88 | 0.03~1.77 | y=2.340x+0.134 | 0.971 | |
| V. mali | 3 | 30.74 | 27.42~34.90 | y=5.375x-7.996 | 0.999 |
| 14 | 15.90 | 13.82~18.40 | y=3.576x-4.296 | 0.980 | |
| Boscalid | 13.44 | 11.55~16.13 | y=3.804x-4.292 | 0.978 | |
| S. sclerotiorum | 3 | 12.07 | 10.22~14.20 | y=2.919x-3.156 | 0.988 |
| 8 | 3.66 | 2.57~4.70 | y=2.370x-1.335 | 0.986 | |
| 11 | 8.79 | 7.28~10.46 | y=2.689x-2.538 | 0.988 | |
| 12 | 15.74 | 13.76~18.08 | y=3.830x-4.584 | 0.986 | |
| 14 | 12.38 | 10.80~14.20 | y=3.755x-4.104 | 0.986 | |
| 21 | 22.90 | 20.41~25.89 | y=5.008x-6.810 | 0.993 | |
| 22 | 13.81 | 9.92~19.38 | y=5.166x-5.891 | 0.983 | |
| 24 | 16.59 | 14.38~19.27 | y=3.492x-4.260 | 0.985 | |
| Boscalid | 0.95 | 0.53~1.35 | y=2.052x+0.049 | 0.960 |
| Fungi | Compd. | EC50/(mg•L-1) | 95% confidence interval | Regression equationa | Rb |
|---|---|---|---|---|---|
| B. cinerea | 3 | 32.61 | 29.13~36.99 | y=5.581x-8.446 | 0.998 |
| 5 | 31.60 | 28.24~35.81 | y=5.550x-8.323 | 0.988 | |
| 8 | 13.82 | 12.27~15.58 | y=4.690x-5.349 | 0.989 | |
| 11 | 21.17 | 18.97~23.72 | y=5.422x-7.188 | 0.989 | |
| 12 | 21.81 | 14.06~40.87 | y=3.120x-4.176 | 0.942 | |
| 14 | 15.31 | 13.56~17.32 | y=4.513x-5.347 | 0.994 | |
| 19 | 21.85 | 19.01~25.43 | y=3.941x-5.010 | 0.986 | |
| 21 | 23.36 | 15.85~40.05 | y=3.498x-4.787 | 0.962 | |
| 22 | 19.25 | 17.11~21.77 | y=4.765x-6.120 | 0.995 | |
| 28 | 23.17 | 20.27~26.85 | y=3.974x-5.420 | 0.980 | |
| Boscalid | 0.88 | 0.03~1.77 | y=2.340x+0.134 | 0.971 | |
| V. mali | 3 | 30.74 | 27.42~34.90 | y=5.375x-7.996 | 0.999 |
| 14 | 15.90 | 13.82~18.40 | y=3.576x-4.296 | 0.980 | |
| Boscalid | 13.44 | 11.55~16.13 | y=3.804x-4.292 | 0.978 | |
| S. sclerotiorum | 3 | 12.07 | 10.22~14.20 | y=2.919x-3.156 | 0.988 |
| 8 | 3.66 | 2.57~4.70 | y=2.370x-1.335 | 0.986 | |
| 11 | 8.79 | 7.28~10.46 | y=2.689x-2.538 | 0.988 | |
| 12 | 15.74 | 13.76~18.08 | y=3.830x-4.584 | 0.986 | |
| 14 | 12.38 | 10.80~14.20 | y=3.755x-4.104 | 0.986 | |
| 21 | 22.90 | 20.41~25.89 | y=5.008x-6.810 | 0.993 | |
| 22 | 13.81 | 9.92~19.38 | y=5.166x-5.891 | 0.983 | |
| 24 | 16.59 | 14.38~19.27 | y=3.492x-4.260 | 0.985 | |
| Boscalid | 0.95 | 0.53~1.35 | y=2.052x+0.049 | 0.960 |

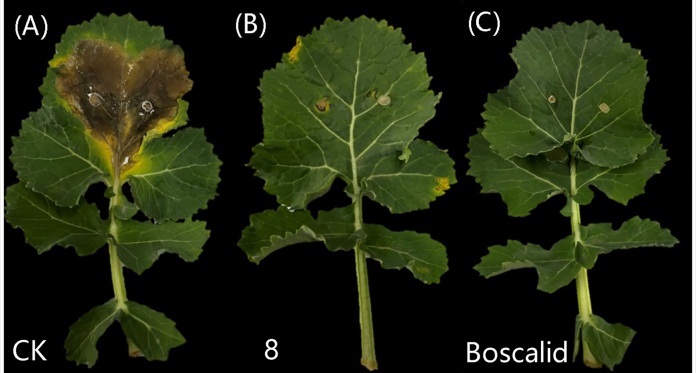
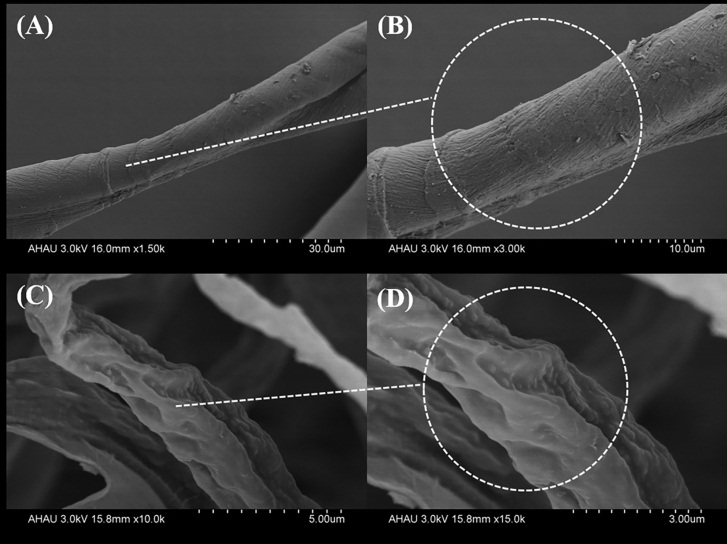
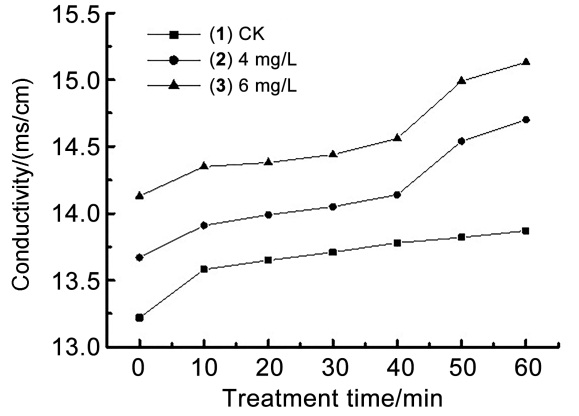
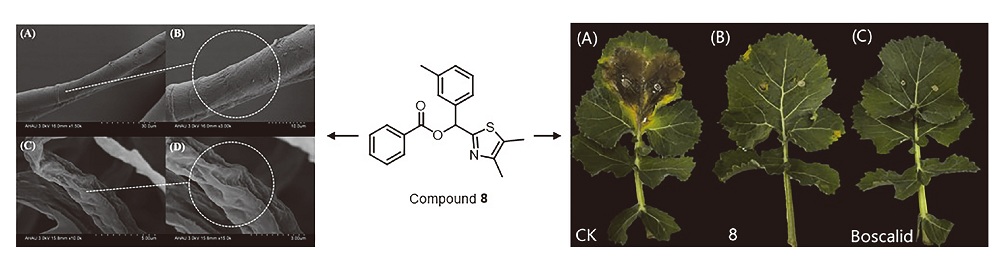
| [1] |
Bolton, M. D.; Thomma, B. P. H. J.; Nelson, B. D. Mol. Plant. Pathol. 2006, 7. 1.
|
| [2] |
Huang, X.-P.; Song, Y.-F.; Li, B.-X.; Mu, W.; Liu, F. Crop Prot. 2019, 122, 42.
doi: 10.1016/j.cropro.2019.04.010 |
| [3] |
Zhu, J.-K.; Gao, J-M.; Yang, C.-J.; Shang, X.-F.; Zhao, Z.-M.; Lawoe, R.-K.; Zhou, R.; Sun, Y.; Yin, X.-D.; Liu, Y. Q. J. Agric. Food Chem. 2020, 68, 2306.
doi: 10.1021/acs.jafc.9b06793 |
| [4] |
Cheng, X.; Wang, W.; Wang, Y.-X.; Xia, D.-G.; Yin, F.; Liu, Q.; Luo, H.; Li, M.; Zhang, C.-Q.; Cao, H.-Q.; Lv, X.-H. J. Agric. Food Chem. 2021, 69, 11395.
doi: 10.1021/acs.jafc.1c02454 |
| [5] |
Yao, T.-T.; Fang, S.-W.; Li, Z.-S.; Xiao, D.-X.; Cheng, J.-L.; Ying, H.-Z.; Du, Y.-J.; Zhao, J.-H.; Dong, X.-W. J. Agric. Food Chem. 2017, 65, 3204.
doi: 10.1021/acs.jafc.7b00249 |
| [6] |
Wang, W.; Zhang, S.; Wang, J.-H.; Wu, F.-R.; Wang, T.; Xu, G. J. Agric. Food Chem. 2021, 69, 491.
doi: 10.1021/acs.jafc.0c06700 |
| [7] |
Wang, X.; Wang, A.; Qiu, L.; Chen, M.; Lu, A.; Li, G.; Yang, C.; Xue, W. J. Agric. Food Chem. 2020, 68, 14426.
doi: 10.1021/acs.jafc.0c03736 |
| [8] |
Wu, Z.; Park, H.-Y.; Xie, D.; Yang, J.; Hou, S.; Shahzad, N.; Kim, C. K.; Yang, S. J. Agric. Food Chem. 2021, 69, 1214.
doi: 10.1021/acs.jafc.0c05702 |
| [9] |
Guo, X.; Zhao, B.; Fan, Z.; Yang, D.-Y.; Belskaya, N. P. J. Agric. Food Chem. 2019, 67, 1647.
doi: 10.1021/acs.jafc.8b06935 |
| [10] |
Chen, L.; Zhao, Bin.; Fan, Z.-J.; Hu, M.-X.; Li, Q.; Hu, W.-H.; Li, J.-W.; Zhang, J.-L. J. Agric. Food Chem. 2019, 67, 12357.
doi: 10.1021/acs.jafc.9b03891 |
| [11] |
Lu, Q.; Yu, Q.; Zhu, Y.-B.; Weng, J.-Q.; Yuan, J.; Hu, D.-X.; Chen, J.; Liu, X.-H.; Tan, C.-X. J. Mol. Struct. 2019, 1180, 780.
doi: 10.1016/j.molstruc.2018.12.068 |
| [12] |
Wu, Q.; Zhao, B.; Fan, Z.-J.; Guo, X.-F.; Yang, D.-Y.; Zang, N.-L.; Yu, B.; Zhou, S.; Zhao, J.-B.; Chen, F. J. Agric. Food Chem. 2019, 67, 1360.
doi: 10.1021/acs.jafc.8b06054 |
| [13] |
Yu, B.; Zhou, S.; Cao, L.-X.; Hao, Z.-S.; Yang, D.-Y.; Guo, X.-F.; Zhang, N.-L.; Bakulev, V.A.; Fan, Z.-J. J. Agric. Food Chem. 2020, 68, 7093.
doi: 10.1021/acs.jafc.0c00062 |
| [14] |
Jr, H; Phares, H.F. Otto, E.J.J.o.I.D. J. Invest. Dermatol. 1964, 42, 479.
doi: 10.1038/jid.1964.101 |
| [15] |
Yang, Y.; Dong, F.-S.; Liu, X.-G.; Xu, J.; Wu, X.-H.; Zheng, Y.-Q. Environ. Pollut. 2020, 265, 115031.
doi: 10.1016/j.envpol.2020.115031 |
| [16] |
Kim, D. S.; Chun, S. J.; Jeon, J. J.; Lee, S. W.; Joe, G. H. Pest. Manage. Sci. 2004, 60, 1007.
doi: 10.1002/ps.873 |
| [17] |
Cohen, Y. J.; Rubin, A. E.; Galperin, M. Phytoparasitica 2018, 46, 689.
doi: 10.1007/s12600-018-0702-6 |
| [18] |
Gao, Y.-Y.; Zhao, X.-J.; Sun, X.-F.; Wang, Z.; Zhang, J.; Li, L.-S.; Shi, H.-Y.; Wang, M.-H. J. Agric. Food Chem. 2021, 69, 3289.
doi: 10.1021/acs.jafc.0c04163 |
| [19] |
Siegenthaler, T. B.; Hansen, Z. Plant Dis. 2021, 105, 1943.
|
| [20] |
Guo, Y.; Fan, J.-P.; Zhang, Q.; Bao, C.-N.; Liu, Z.-Y.; Yang, R. G. Bioorg. Med. Chem. Lett. 2019, 29, 179.
doi: 10.1016/j.bmcl.2018.12.002 |
| [21] |
Soliman, N. N.; Salam, M. A. E.; Fadda, A. A.; Abdel-Motaal, M. J. Agric. Food Chem. 2020, 68,5790.
|
| [22] |
Liu, H.; Xia, D.-G.; Chu, Z.-W.; Hu, R.; Lv, X.-H. Bioorg. Chem. 2020, 100, 103907.
doi: 10.1016/j.bioorg.2020.103907 |
| [23] |
Mattson, A. E.; Bharadwaj, A. R.; Zuhl, A. M.; Scheidt, K. A. J. Org. Chem. 2006, 71. 5715.
|
| [24] |
Mattson, A. E.; Zuhl, A. M.; Reynolds, T. E.; Scheidt, K. A. J. Am. Chem. Soc. 2006, 128, 4932.
pmid: 16608309 |
| [25] |
Ren, Z.-L.; Liu, H.; Jiao, D.; Hu, H.-T.; Wang, Wei.; Gong, J.-X.; Wang, A.-L.; Cao, H.-Q.; Lv, X.-H. Drug Dev. Res. 2018, 79, 307.
doi: 10.1002/ddr.21469 |
| [26] |
Liu, H.; Xia, D.-G.; Hu, R.; Wang, W.; Cheng, X.; Wang, A.-L.; Zhang, Q.; Lv, X.-H. Pestic. Biochem. Phys. 2020, 163, 271.
doi: 10.1016/j.pestbp.2019.11.024 |
| [27] |
Wang, W.; Cheng, X.; Cui, X.; Xia, D.-G.; Wang, Z.-Q.; Lv, X.-H. Pest. Manage. Sci. 2021, 77, 3529.
doi: 10.1002/ps.6406 |
| [28] |
Yin, X.-D.; Ma, K.-Y.; Wang, Y.-L.; Sun, Y.; Shang, X.-F.; Zhao, Z.-M.; Wang, R.-X; Chen, Y.-J.; Zhu, J.-K.; Liu, Y.-Q. J. Agric. Food Chem. 2020, 68, 11096.
doi: 10.1021/acs.jafc.0c01322 |
| [29] |
Magdalena, E.-S.; Agnieszka, N.; Agata, C.; Monika, Ś.; Anna, O.; Dorota, Ż. Food Chem. 2021, 350, 129218.
doi: 10.1016/j.foodchem.2021.129218 |
| [30] |
Sabatino, V.; Rebelein, J. G.; Ward, T. R. J. Am. Chem. Soc. 2019, 141, 17048.
doi: 10.1021/jacs.9b07193 pmid: 31503474 |
| [1] | 贾小英, 普佳霞, 韩丽荣, 李清寒. 含双杂原子苯并[d]五元杂环硫醚类化合物的合成研究进展[J]. 有机化学, 2024, 44(1): 18-40. |
| [2] | 钟玉梅, 邹小颖, 卓小丫, 王逸涵, 申佳奕, 郑绿茵, 郭维. 4-氧代-2-亚胺基噻唑烷-5-亚基乙酸乙酯类化合物的设计、合成及抗癌活性[J]. 有机化学, 2023, 43(4): 1452-1461. |
| [3] | 张越华, 聂飞, 周路, 王晓烽, 刘源, 霍延平, 陈文铖, 赵祖金. 苯并噻唑酮类热活化延迟荧光材料的合成及其光电性能研究[J]. 有机化学, 2023, 43(11): 3876-3887. |
| [4] | 刘梦, 黄延茹, 孙小飞, 汤立军. 一种基于“聚集诱导发光+激发态分子内质子转移”机制的苯并噻唑衍生物荧光探针及其对次氯酸根的识别[J]. 有机化学, 2023, 43(1): 345-351. |
| [5] | 乃比江•赛米, 张蕾, 买地娜•沙拉木, 曾竟, 阿布都热西提•阿布力克木. 硫代磺酸酯和磺酰卤的绿色合成研究[J]. 有机化学, 2023, 43(1): 236-243. |
| [6] | 黄兴, 张昌浩, 邓浩, 沈庆坤, 郭红艳, 全哲山, 李志勇, 金莉莉. 常春藤皂苷元衍生物的合成和抗肿瘤活性[J]. 有机化学, 2022, 42(9): 2877-2887. |
| [7] | 陈伟, 雷思敏, 兰雨欣, 许豪键, 余坪槟, 张锐, 吴润, 陈阳. 新型喹唑啉酮衍生物的设计合成与抗植物病原真菌活性研究[J]. 有机化学, 2022, 42(7): 2164-2171. |
| [8] | 王伟, 武复冉, 马一丹, 徐丹, 徐功. 含取代吡唑新型苯甲酰胺类化合物的合成及抗真菌活性研究[J]. 有机化学, 2022, 42(2): 607-618. |
| [9] | 戴洪林, 司晓杰, 池玲玲, 王浩, 高潮, 汪正捷, 刘丽敏, 马家婕, 于富强, 刘宏民, 可钰, 张秋荣. 含噻唑结构的2,4,6-三取代喹唑啉衍生物的合成及抗肿瘤活性研究[J]. 有机化学, 2022, 42(11): 3853-3862. |
| [10] | 陈澍, 邵莹莹, 付欣豪, 陈庆悟, 杜晓华, 谭成侠. 吡啶联噻唑双酰胺类化合物的设计、合成及杀虫活性[J]. 有机化学, 2022, 42(11): 3870-3879. |
| [11] | 傅琴姣, 张瑞芹, 裘浣沂, 马仁超, 马永敏. Selectfluor氧化合成2-芳基苯并噻唑的新方法[J]. 有机化学, 2021, 41(9): 3585-3592. |
| [12] | 陆棋, 叶飞霞, 孙晓彤, 翁建全, 余茜, 胡德玄. 新型拟天然芪类拓扑异构酶I抑制剂的设计与合成[J]. 有机化学, 2021, 41(8): 3321-3329. |
| [13] | 董金娇, 朱昕悦, 冯思冉, 张超超, 刘振明, 乔晓强, 宋亚丽. 7-苯基-6H,7H-1,3,4-噻二唑并[3,2-a]-硫色烯并[4,3-d]嘧啶类化合物的合成及抗真菌活性研究[J]. 有机化学, 2021, 41(6): 2467-2475. |
| [14] | 张涛, 李念先, 周楠茜, 马雯, 卫海沅, 张冰欣, 陈亮辉, 海广范, 段迎超, 白素平. 新型蓝萼甲素-噻唑类衍生物的设计、合成与生物学评价[J]. 有机化学, 2021, 41(6): 2393-2400. |
| [15] | 张晓平, 金桂勇, 陈芝飞, 王清福, 赵森森, 武志勇, 万帅, 席高磊, 赵旭. 吡嗪-噻唑联芳类化合物的合成及抗氧化性能研究[J]. 有机化学, 2021, 41(6): 2445-2453. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||