有机化学 ›› 2023, Vol. 43 ›› Issue (8): 2614-2627.DOI: 10.6023/cjoc202302010 上一篇 下一篇
综述与进展
王玉超a,b,c, 刘晋彪a,*( ), 何智涛b,c,*(
), 何智涛b,c,*( )
)
收稿日期:2023-02-11
修回日期:2023-03-18
发布日期:2023-04-07
基金资助:
Yuchao Wanga,b,c, Jinbiao Liua( ), Zhitao Heb,c(
), Zhitao Heb,c( )
)
Received:2023-02-11
Revised:2023-03-18
Published:2023-04-07
Contact:
*Supported by:文章分享

由于原料来源广泛、高原子经济性和高立体选择性控制等优势, 钯催化共轭二烯的不对称氢官能团化已发展成为一种高效合成手性烯丙基片段的方法, 包括构建烯丙基C—C、C—N、C—S、C—P、C—Si和C—O键等. 总结了近几十年来该领域的发展现状、最新进展以及机制特点等. 根据所构建的化学键的不同, 主要涵盖了不对称氢烷基化、氢胺化、氢膦化、氢砜化、氢硅化和氢醚化六个部分.
王玉超, 刘晋彪, 何智涛. 钯催化共轭二烯的不对称氢官能团化[J]. 有机化学, 2023, 43(8): 2614-2627.
Yuchao Wang, Jinbiao Liu, Zhitao He. Palladium-Catalyzed Asymmetric Hydrofunctionalizations of Conjugated Dienes[J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2614-2627.
| [1] |
For reviews on transition metal-catalyzed asymmetric allylation, see (a) Trost, B. M.; Van Vranken, D. L. Chem. Rev. 1996, 96, 395.
doi: 10.1021/cr9409804 pmid: 33570909 |
|
(b) Trost, B. M.; Crawley, M. L. Chem. Rev. 2003, 103, 2921.
doi: 10.1021/cr020027w pmid: 33570909 |
|
|
(c) Lu, Z.; Ma, S. Angew. Chem., Int. Ed. 2008, 47, 258.
pmid: 33570909 |
|
|
(d) Butt, N. A.; Zhang, W. Chem. Soc. Rev. 2015, 44, 7929.
doi: 10.1039/C5CS00144G pmid: 33570909 |
|
|
(e) Süsse, L.; Stoltz, B. M. Chem. Rev. 2021, 121, 4084.
doi: 10.1021/acs.chemrev.0c01115 pmid: 33570909 |
|
| [2] |
For selected reviews on Ir-catalyzed asymmetric allylic substitution, see (a) Helmchen, G. J. Organomet. Chem. 1999, 576, 203.
doi: 10.1016/S0022-328X(98)01059-6 pmid: 33739109 |
|
(b) Graening, T.; Schmalz, H.-G. Angew. Chem., Int. Ed. 2003, 42, 2580.
doi: 10.1002/anie.200301644 pmid: 33739109 |
|
|
(c) Trost, B. M. Tetrahedron 2015, 71, 5708.
doi: 10.1016/j.tet.2015.06.044 pmid: 33739109 |
|
|
(d) Pàmies, O.; Margalef, J.; Cañellas, S.; James, J.; Judge, E.; Guiry, P. J.; Moberg, C.; Bäckvall, J.-E.; Pfaltz, A.; Pericàs, M. A.; Diéguez, M. Chem. Rev. 2021, 121, 4373.
doi: 10.1021/acs.chemrev.0c00736 pmid: 33739109 |
|
| [3] |
For selected reviews on Ir-catalyzed asymmetric allylic substitution, see (a) Hartwig, J. F.; Pouy, M. J. Top. Organomet. Chem. 2011, 34, 169.
pmid: 28649462 |
|
(b) Hethcox, J. C.; Shockley, S. E.; Stoltz, B. M. ACS Catal. 2016, 6, 6207.
doi: 10.1021/acscatal.6b01886 pmid: 28649462 |
|
|
(c) Qu, J.; Helmchen, G. Acc. Chem. Res. 2017, 50, 2539.
doi: 10.1021/acs.accounts.7b00300 pmid: 28649462 |
|
|
(d) Cheng, Q.; Tu, H. F.; Zheng, C.; Qu, J. P.; Helmchen, G.; You, S. L. Chem. Rev. 2019, 119, 1855.
doi: 10.1021/acs.chemrev.8b00506 pmid: 28649462 |
|
|
(e) Rössler, S. L.; Petrone, D. A.; Carreira, E. M. Acc. Chem. Res. 2019, 52, 2657.
doi: 10.1021/acs.accounts.9b00209 pmid: 28649462 |
|
| [4] |
For selected reviews on Ir-catalyzed asymmetric allylic substitution, see (a) Evans, P. A.; Leahy, D. K. In Modern Rhodium-Cata- lyzed Organic Reactions, Ed.: Evans, P. A., Wiley-VCH, Weinheim, Germany, 2005, Chapter 10, pp. 191-214.
pmid: 30183287 |
|
(b) Turnbull, B. W. H.; Evans, P. A. J. Org. Chem. 2018, 83, 11463.
doi: 10.1021/acs.joc.8b00583 pmid: 30183287 |
|
|
(c) Thoke, M. B.; Kang, Q. Synthesis 2019, 51, 2585.
doi: 10.1055/s-0037-1611784 pmid: 30183287 |
|
| [5] |
For representative work on Ru-catalyzed asymmetric allylic substitution, see (a) Kanbayashi, N.; Onitsuka, K. J. Am. Chem. Soc. 2010, 132, 1206.
doi: 10.1021/ja908456b pmid: 20052978 |
|
(b) Trost, B. M.; Rao, M.; Dieskau, A. P. J. Am. Chem. Soc. 2013, 135, 18697.
doi: 10.1021/ja411310w pmid: 20052978 |
|
| [6] |
For a selected review on Cu-catalyzed asymmetric allylic substitution, see: Alexakis, A.; Bäckvall, J. E.; Krause, N.; Pàmies, O.; Diéguez, M. Chem. Rev. 2008, 108, 2796.
doi: 10.1021/cr0683515 pmid: 18671436 |
| [7] |
For a selected review on Mo-catalyzed asymmetric allylic substitution, see: Belda, O.; Moberg, C. Acc. Chem. Res. 2004, 37, 159.
doi: 10.1021/ar030239v |
| [8] |
For a representative work on Ni-catalyzed asymmetric allylic substitution, see: Liu, X.-T.; Zhang, Y.-Q.; Han, X.-Y.; Sun, S.-P.; Zhang, Q.-W. J. Am. Chem. Soc. 2019, 141, 16584.
doi: 10.1021/jacs.9b08734 |
| [9] |
For a representative work on Co-catalyzed asymmetric allylic substitution, see: Ghorai, S.; Chirke, S. S.; Xu, W.-B.; Chen, J.-F.; Li, C. J. Am. Chem. Soc. 2019, 141, 11430.
doi: 10.1021/jacs.9b06035 pmid: 31274311 |
| [10] |
For reviews on allylic C-H functionalizations, see (b) Wang, R.; Luan, Y.; Ye, M. Chin. J. Chem. 2019, 37, 720.
doi: 10.1002/cjoc.v37.7 |
|
(b) Wang, P.-S.; Gong, L.-Z. Acc. Chem. Res. 2020, 53, 2841.
doi: 10.1021/acs.accounts.0c00477 |
|
| [11] |
(a) Li, G.; Huo, X.; Jiang, X.; Zhang, W. Chem. Soc. Rev. 2020, 49, 2060.
doi: 10.1039/C9CS00400A |
|
(b) Koschker, P.; Breit, B. Acc. Chem. Res. 2016, 49, 1524.
doi: 10.1021/acs.accounts.6b00252 |
|
|
(c) Ma, C.; Chen, Y.-W.; He, Z.-T. Sci. Sin. Chim. 2023, 53, 474.
doi: 10.1360/SSC-2022-0196 |
|
| [12] |
(a) Adamson, N. J.; Malcolmson, S. J. ACS Catal. 2020, 10, 1060.
doi: 10.1021/acscatal.9b04712 |
|
(b) Flaget, A.; Zhang, C.; Mazet, C. ACS Catal. 2022, 12, 15638.
doi: 10.1021/acscatal.2c05251 |
|
| [13] |
(a) Zhang, Q.; Dong, D.; Zi, W. J. Am. Chem. Soc. 2020, 142, 15860.
doi: 10.1021/jacs.0c05976 |
|
(b) Jiu, A. Y.; Slocumb, H. S.; Yeung, C. S.; Yang, X.-H.; Dong, V. M. Angew. Chem., Int. Ed. 2021, 60, 19660.
doi: 10.1002/anie.v60.36 |
|
| [14] |
(a) Takacs, J. M.; Lawson, E. C.; Clement, F. J. Am. Chem. Soc. 1997, 119, 5956.
doi: 10.1021/ja962313t pmid: 31442059 |
|
(b) Bernar, I.; Fiser, B.; Blanco-Ania, D.; Gómez-Bengoa, E.; Rutjes, F. P. J. T. Org. Lett. 2017, 19, 4211.
doi: 10.1021/acs.orglett.7b01826 pmid: 31442059 |
|
|
(c) Tsukamoto, H.; Konno, T.; Ito, K.; Doi, T. Org. Lett. 2019, 21, 6811.
doi: 10.1021/acs.orglett.9b02439 pmid: 31442059 |
|
| [15] |
(a) Csákÿ, A. G.; de la Herrán, G.; Murcia, M. C. Chem. Soc. Rev. 2010, 39, 4080.
doi: 10.1039/b924486g |
|
(b) Hussain, Y.; Tamanna; Sharma, M.; Kumar, A.; Chauhan, P. Org. Chem. Front. 2022, 9, 572.
doi: 10.1039/D1QO01561C |
|
|
(c) Chauhan, P.; Kaya, U.; Enders, D. Adv. Synth. Catal. 2017, 359, 888.
doi: 10.1002/adsc.201601342 |
|
| [16] |
(a) Hata, G.; Takahashi, K.; Miyake, A. J. Org. Chem. 1971, 36, 2116.
doi: 10.1021/jo00814a018 |
|
(b) Takahashi, K.; Miyake, A.; Hata, G. Bull. Chem. Soc. Jpn. 1972, 45, 1183.
doi: 10.1246/bcsj.45.1183 |
|
| [17] |
(a) Jolly, P. W.; Kokel, N. Synthesis 1990, 771.
|
|
(b) Trost, B. M.; Zhi, L. Tetrahedron Lett. 1992, 33, 1831.
doi: 10.1016/S0040-4039(00)74154-2 |
|
|
(c) Goddard, R.; Hopp, G.; Jolly, P. W.; Kriiger, C.; Mynott, R.; Wirtz, C. J. Organomet. Chem. 1995, 486, 163.
doi: 10.1016/0022-328X(94)05017-6 |
|
| [18] |
Leitner, A.; Larsen, J.; Steffens, C.; Hartwig, J. F. J. Org. Chem. 2004, 69, 7552.
doi: 10.1021/jo0490999 |
| [19] |
Adamson, N. J.; Wilbur, K. C. E.; Malcolmson, S. J. J. Am. Chem. Soc. 2018, 140, 2761.
doi: 10.1021/jacs.7b13300 pmid: 29446922 |
| [20] |
Park, S.; Adamson, N. J.; Malcolmson, S. J. Chem. Sci. 2019, 10, 5176.
doi: 10.1039/C9SC00633H |
| [21] |
Adamson, N. J.; Park, S.; Zhou, P.-F.; Nguyen, A. L.; Malcolmson, S. J. Org. Lett. 2020, 22, 2032.
doi: 10.1021/acs.orglett.0c00412 pmid: 32052974 |
| [22] |
Zhang, Z.-P.; Xiao, F.; Wu, H.-M.; Dong, X.-Q.; Wang, C.-J. Org. Lett. 2020, 22, 569.
doi: 10.1021/acs.orglett.9b04341 |
| [23] |
Yang, H.-J.; Xing, D. Chem. Commun. 2020, 56, 3721.
doi: 10.1039/D0CC00265H |
| [24] |
Onyeagusi, C. I.; Shao, X.-X.; Malcolmson, S. J. Org. Lett. 2020, 22, 1681.
doi: 10.1021/acs.orglett.0c00342 pmid: 32013445 |
| [25] |
(a) Zhang, Q.-L.; Yu, H.-M.; Shen, L.-L.; Tang, T.-H.; Dong, D.-F.; Chai, W.-W.; Zi, W.-W. J. Am. Chem. Soc. 2019, 141, 14554.
doi: 10.1021/jacs.9b07600 |
|
(b) Wang, H.-F.; Zhang, R.-Y.; Zhang, Q.-L.; Zi, W.-W. J. Am. Chem. Soc. 2021, 143, 10948.
doi: 10.1021/jacs.1c02220 |
|
|
(c) Zhang, Q.-L.; Zhu, M.-H.; Zi, W.-W. Chem 2022, 8, 2784.
doi: 10.1016/j.chempr.2022.07.014 |
|
|
(d) Han, J.-Q.; Liu, R.-X.; Lin, Z.-T.; Zi, W.-W. Angew. Chem., Int. Ed. 2023, 62, e202215714.
doi: 10.1002/anie.v62.2 |
|
| [26] |
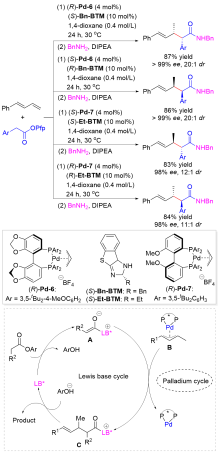
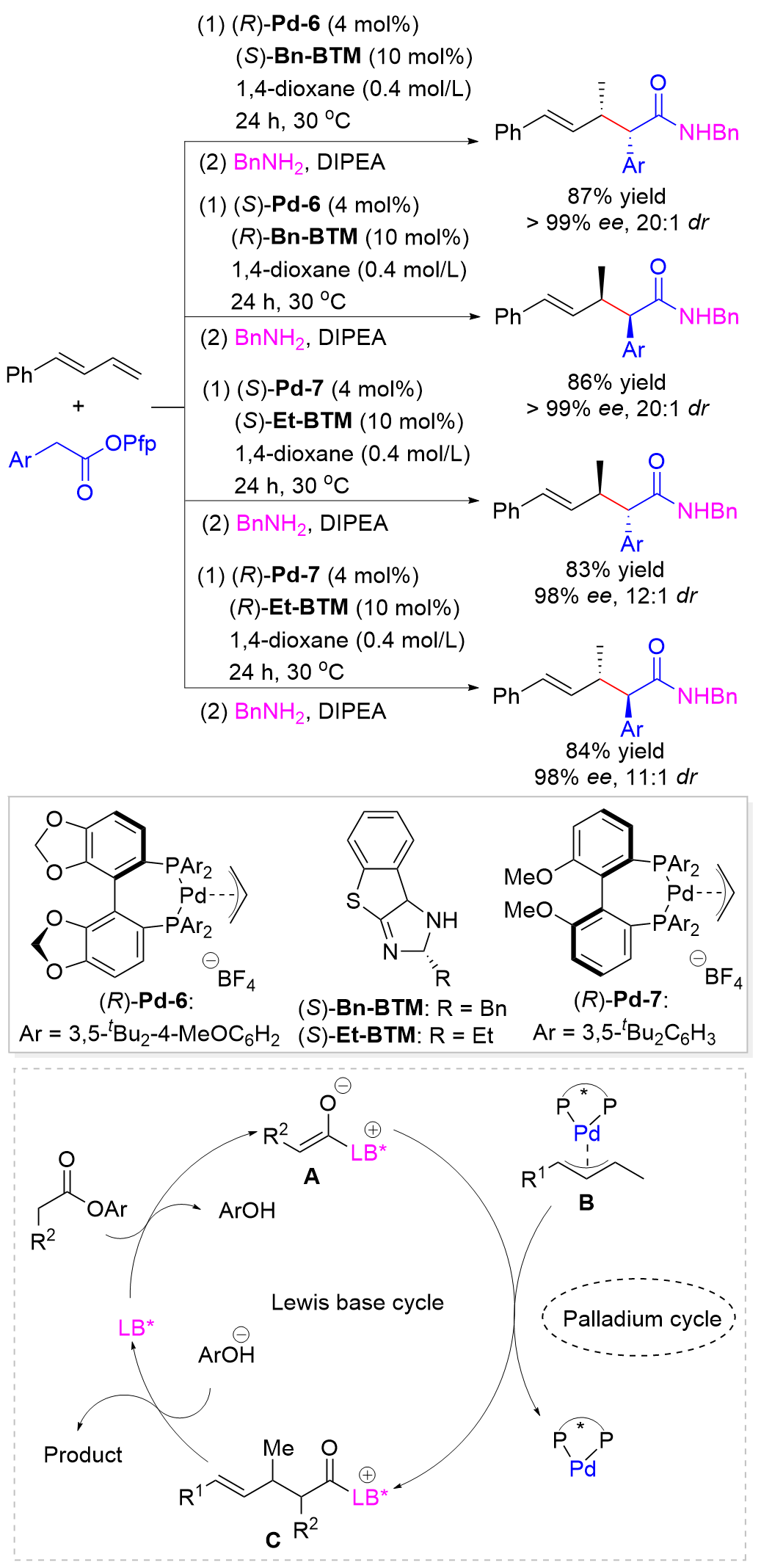
Wang, Y.-C.; Xiao, Z.-X.; Wang, M.; Yang, S.-Q.; Liu, J.-B.; He, Z.-T. Angew. Chem., Int. Ed. 2022, 61, e202215568.
|
| [27] |
(a) Krautwald, S.; Sarlah, D.; Schafroth, M. A.; Carreira, E. M. Science 2013, 340, 1065.
doi: 10.1126/science.1237068 pmid: 29676895 |
|
(b) Krautwald, S.; Carreira, E. M. J. Am. Chem. Soc. 2017, 139, 5627.
doi: 10.1021/jacs.6b13340 pmid: 29676895 |
|
|
(c) Beletskaya, I. P.; Najera, C.; Yus, M. Chem. Rev. 2018, 118, 5080.
doi: 10.1021/acs.chemrev.7b00561 pmid: 29676895 |
|
|
(d) Kalita, S. J.; Huang, Y. Y.; Schneider, U. Sci. Bull. 2020, 65, 1865.
doi: 10.1016/j.scib.2020.08.005 pmid: 29676895 |
|
|
(e) Huo, X.; Li, G.; Wang, X.; Zhang, W. Angew. Chem., Int. Ed. 2022, 61, e202210086.
doi: 10.1002/anie.v61.45 pmid: 29676895 |
|
| [28] |
Armbruster, R. W.; Morgan, M. M.; Schmidt, J. L.; Lau, C. M.; Riley, R. M.; Zabrowsky, D. L.; Dieck, H. A. Organometallics 1986, 5, 234.
doi: 10.1021/om00133a011 |
| [29] |
Minami, T.; Okamoto, H.; Ikeda, S.; Tanaka, R.; Ozawa, F.; Yoshifuji, M. Angew. Chem., Int. Ed. 2001, 40, 4501.
doi: 10.1002/1521-3773(20011203)40:23【-逻*辑*与-】amp;lt;4501::AID-ANIE4501【-逻*辑*与-】amp;gt;3.0.CO;2-K |
| [30] |
Löber, O.; Kawatsura, M.; Hartwig, J. F. J. Am. Chem. Soc. 2001, 123, 4366.
pmid: 11457216 |
| [31] |
Adamson, N. J.; Hull, E.; Malcolmson, S. J. J. Am. Chem. Soc. 2017, 139, 7180.
doi: 10.1021/jacs.7b03480 pmid: 28453290 |
| [32] |
Park, S.; Malcolmson, S. J. ACS Catal. 2018, 8, 8468.
doi: 10.1021/acscatal.8b01914 |
| [33] |
Jiu, A. Y.; Slocumb, H. S.; Yeung, C. S.; Yang, X.-H.; Dong, V. M. Angew. Chem., Int. Ed. 2021, 60, 19660.
doi: 10.1002/anie.v60.36 |
| [34] |
(a) Wei, X.; Lu, J.-Z.; Duan, W.-L. Synthesis 2016, 48, 4155.
doi: 10.1055/s-0035-1562456 |
|
(b) Yang, Z.-P.; Wang, J. J. Angew, Chem., Int. Ed. 2021, 60, 27288.
|
|
|
(c) Long, J.; Li, Y.-Q.; Zhao, W.-N.; Yin, G.-Y. Chem. Sci. 2022, 13, 1390.
doi: 10.1039/D1SC05651D |
|
| [35] |
Hirao, T.; Masunaga, T.; Yamada, N.; Ohshiro, Y.; Agawa, T. Bull. Chem. Soc. Jpn. 1982, 55, 909.
doi: 10.1246/bcsj.55.909 |
| [36] |
Mirzaei, F.; Han, L.-B.; Tanaka, M. Tetrahedron Lett. 2001, 42, 297.
doi: 10.1016/S0040-4039(00)01928-6 |
| [37] |
Nie, S.-Z.; Davison, R. T.; Dong, V. M. J. Am. Chem. Soc. 2018, 140, 16450.
doi: 10.1021/jacs.8b11150 |
| [38] |
Zhang, Q.-L.; Dong, D.-F.; Zi, W.-W. J. Am. Chem. Soc. 2020, 142, 15860.
doi: 10.1021/jacs.0c05976 |
| [39] |
Li, M.-M.; Cheng, L.; Xiao, L.-J.; Xie, J.-H.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2021, 60, 2948.
doi: 10.1002/anie.v60.6 |
| [40] |
Hayashi, T.; Kabeta, K.; Yamamoto, T.; Tamao, K.; Kumada, M. Tetrahedron Lett. 1983, 24, 5661.
doi: 10.1016/S0040-4039(00)94167-4 |
| [41] |
Okada, T.; Morimoto, T.; Achiwa, K. Chem. Lett. 1990, 999.
|
| [42] |
Gustafsson, M.; Bergqvist, K.-E.; Frejd, T. J. Chem. Soc., Perkin Trans. 1, 2001, 1452.
|
| [43] |
Kitayama, K.; Tsuji, H.; Uozumi, Y.; Hayashi, T. Tetrahedron Lett. 1996, 37, 4169.
doi: 10.1016/0040-4039(96)00786-1 |
| [44] |
Hayashi, T.; Han, J. W.; Takeda, A.; Tang, J.; Nohmi, K.; Mukaide, K.; Tsuji, H.; Uozumi, Y. Adv. Synth. Catal. 2001, 343, 279.
doi: 10.1002/(ISSN)1615-4169 |
| [45] |
Han, J. W.; Hayashi, T. Chem. Lett. 2001, 976.
|
| [46] |
Park, H. S.; Han, J. W.; Shintani, R.; Hayashi, T. Tetrahedron: Asymmetry 2013, 24, 418.
|
| [47] |
Hayashi, T.; Kabeta, K. Tetrahedron Lett. 1985, 26, 3023.
|
| [48] |
Han, J.-W.; Hayashi, T. Tetrahedron: Asymmetry 2002, 13, 325.
|
| [49] |
Han, J. W.; Tokunaga, N.; Hayashi, T. Helv. Chim. Acta 2002, 85, 3848.
doi: 10.1002/1522-2675(200211)85:11【-逻*辑*与-】amp;lt;3848::AID-HLCA3848【-逻*辑*与-】amp;gt;3.0.CO;2-V |
| [50] |
Yang, S.-Q.; Han, A.-J.; Liu, Y.; Tang, X.-Y.; Lin, G.-Q.; He, Z.-T. J. Am. Chem. Soc. 2023, 145, 3915.
doi: 10.1021/jacs.2c11843 |
| [51] |
Chen, Y.-W.; Liu, Y.; Lu, H.-Y.; Lin, G.-Q.; He, Z.-T. Nat. Commun. 2021, 12, 5626.
doi: 10.1038/s41467-021-25978-6 |
| [1] | 孟宪强, 杨艺, 梁万洁, 王靖涛, 张荣葵, 刘会. 钯催化联烯胺区域选择性芳基酚氧化反应[J]. 有机化学, 2024, 44(1): 224-231. |
| [2] | 王化坤, 任晓龙, 宣宜宁. 卤盐催化的α,β-环氧羧酸酯与异氰酸酯[3+2]环加成反应研究[J]. 有机化学, 2024, 44(1): 251-258. |
| [3] | 于士航, 刘嘉威, 安碧玉, 边庆花, 王敏, 钟江春. 黑腹尼虎天牛接触性信息素的不对称合成[J]. 有机化学, 2024, 44(1): 301-308. |
| [4] | 王兢睿, 冯永奎, 王能中, 黄年玉, 姚辉. 钯催化立体选择性合成硝基烷类β-碳糖苷[J]. 有机化学, 2023, 43(9): 3216-3225. |
| [5] | 徐光利, 许静, 徐海东, 崔香, 舒兴中. 过渡金属催化烯烃和炔烃合成1,3-共轭二烯化合物研究进展[J]. 有机化学, 2023, 43(6): 1899-1933. |
| [6] | 宋亭谕, 李冉, 黄利华, 贾世琨, 梅光建. N—N单键阻转异构体的催化不对称合成[J]. 有机化学, 2023, 43(6): 1977-1990. |
| [7] | 罗诚, 尹艳丽, 江智勇. P-手性膦氧化物的不对称合成研究进展[J]. 有机化学, 2023, 43(6): 1963-1976. |
| [8] | 李落墨, 杨小会. 离子转移反应的研究进展[J]. 有机化学, 2023, 43(3): 1036-1044. |
| [9] | 蒙玲, 汪君. 硫代黄烷酮类衍生物的合成研究进展[J]. 有机化学, 2023, 43(3): 873-891. |
| [10] | 向勋, 何照林, 董秀琴. 钯和手性磷酸协同催化高效构建手性分子的研究进展[J]. 有机化学, 2023, 43(3): 791-808. |
| [11] | 孙美娇, 谭晶, 谭玉, 彭进松, 陈春霞. 钯催化3-(2-氨基嘧啶-4-基)吲哚2位C—H键芳基化反应的研究[J]. 有机化学, 2023, 43(11): 3945-3959. |
| [12] | 张怀远, 许诺, 唐蓉萍, 石星丽. 手性高价碘试剂诱导的不对称去芳构化反应研究进展[J]. 有机化学, 2023, 43(11): 3784-3805. |
| [13] | 匡鑫, 丁昌华, 吴奕晨, 王鹏. 手性烯丙基硅烷的催化对映选择性合成[J]. 有机化学, 2023, 43(10): 3367-3387. |
| [14] | 濮留洋, 李芷悦, 李利民, 马玉翠, 马民, 胡胜全, 吴正治. 秋水仙碱及其天然类似物(–)-N-乙酰秋水酚甲醚的不对称合成[J]. 有机化学, 2023, 43(1): 313-319. |
| [15] | 熊威, 石斌, 姜烜, 陆良秋, 肖文精. 配体调控钯催化乙烯基环状碳酰胺和异氰酸酯的差异性转化[J]. 有机化学, 2023, 43(1): 265-273. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||