有机化学 ›› 2023, Vol. 43 ›› Issue (12): 4157-4167.DOI: 10.6023/cjoc202305029 上一篇 下一篇
综述与进展
收稿日期:2023-05-24
修回日期:2023-07-11
发布日期:2023-08-16
基金资助:Received:2023-05-24
Revised:2023-07-11
Published:2023-08-16
Contact:
*E-mail: Supported by:文章分享

近年来, N-亚硝基通过与过渡金属螯合协同活化芳烃C(sp2)—H键, 成功构建了C—C键和C—杂原子键的例子已有报道. 它基于内部N—N键的氧化断裂, 在反应过程中无需添加外部氧化剂, 反应结束后可自行离去, 发展成为一种新型高效的导向基团, 受到了研究者的广泛关注. 总结了基于N-亚硝基导向的芳烃C(sp2)—H键官能团化的最新研究进展.
王芳, 王磊. 基于N-亚硝基导向的芳烃C(sp2)—H键官能团化研究进展[J]. 有机化学, 2023, 43(12): 4157-4167.
Fang Wang, Lei Wang. Recent Advances in Functionalization of Aromatic C(sp2)—H Bonds Based on N-Nitroso Direction[J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4157-4167.
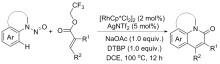
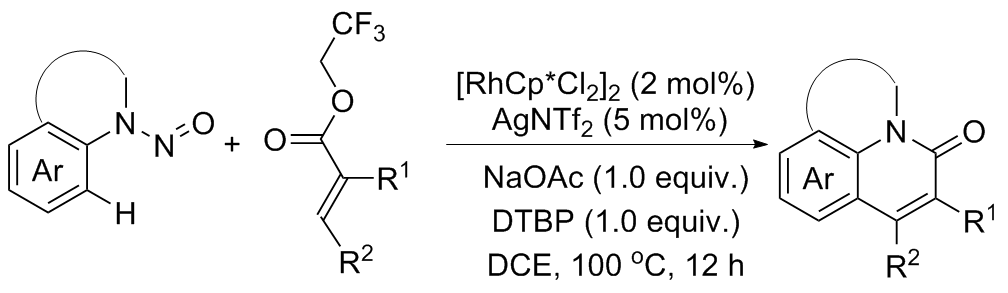
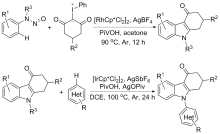

| [1] |
(a) González J. M.; Vidal X.; Ortuño M. A.; Mascareñas J. L.; Gulías M. J. Am. Chem. Soc. 2022, 144, 21437.
doi: 10.1021/jacs.2c09479 pmid: 36378026 |
|
(b) Thombal R. S.; Rubio P. Y. M.; Lee D.; Maiti D.; Lee Y. R. ACS Catal. 2022, 12, 5217.
doi: 10.1021/acscatal.2c00813 pmid: 36378026 |
|
|
(c) Yang S.; Zhang Y. G. Org. Lett. 2022, 24, 9060.
doi: 10.1021/acs.orglett.2c03699 pmid: 36378026 |
|
|
(d) Müller S.; Lee W.; Song J. Y.; Kang E.; Joo J. M. Chem. Commun. 2022, 58, 10809.
doi: 10.1039/D2CC04208H pmid: 36378026 |
|
|
(e) Chen Y.-Z.; Bao G.-Y.; Zhan X.-C.; Fu J.-G.; Ji X.-M.; Zhang S.-S.; Feng C.-G. Chin. J. Chem. 2022, 40, 2188.
doi: 10.1002/cjoc.v40.18 pmid: 36378026 |
|
|
(f) Nale S. D.; Maiti D.; Lee Y. R. Org. Lett. 2021, 23, 2465.
doi: 10.1021/acs.orglett.1c00391 pmid: 36378026 |
|
|
(g) Aslam M.; Mohandoss S.; Lee Y. R. Org. Lett. 2021, 23, 6206.
doi: 10.1021/acs.orglett.1c01793 pmid: 36378026 |
|
|
(h) Wang S. Q.; Miao E.; Wang H.; Song B. C.; Huang W.; Yang W. B. Chem. Commun. 2021, 57, 5929.
doi: 10.1039/D1CC01778K pmid: 36378026 |
|
|
(i) Fu L. Q.; Xu W. Q.; Pu M. P.; Wu Y. D.; Liu Y. Y.; Wan J. P. Org. Lett. 2022, 24, 3003.
doi: 10.1021/acs.orglett.2c00912 pmid: 36378026 |
|
|
(j) Liu Y. Y.; Qu Y. L.; Kang Y. S.; Zhu Y. L.; Sun W. Y.; Lu Y. Org. Lett. 2022, 24, 3118.
doi: 10.1021/acs.orglett.2c00620 pmid: 36378026 |
|
|
(k) Paul T.; Basak S.; Punniyamurthy T. Org. Lett. 2022, 24, 6000.
doi: 10.1021/acs.orglett.2c02265 pmid: 36378026 |
|
|
(l) Raziullah; Kumar M.; Ahmad A.; Dutta H. S.; Rastogi A.; Gangwarc M. K.; Koley D. Chem. Commun. 2022, 58, 3481.
doi: 10.1039/D2CC00257D pmid: 36378026 |
|
|
(m) Mohanty S. R.; Prusty N.; Nanda T.; Banjare S. K.; Ravikumar P. C. J. Org. Chem. 2022, 87, 6189.
doi: 10.1021/acs.joc.2c00428 pmid: 36378026 |
|
|
(n) Shan C. H.; Zhu L.; Qu L. B.; Bai R. P.; Lan Y. Chem. Soc. Rev. 2018, 47, 7552.
doi: 10.1039/C8CS00036K pmid: 36378026 |
|
|
(o) Xu C. F.; Tassone J. P.; Mercado B. Q.; Ellman J. A. Angew. Chem., Int. Ed. 2022, 61, e202202364.
doi: 10.1002/anie.v61.25 pmid: 36378026 |
|
|
(p) Prasad S.; Tantillo D. J. Organometallics 2022, 41, 937.
doi: 10.1021/acs.organomet.1c00662 pmid: 36378026 |
|
|
(q) Chen H. Y.; Wang L. L.; Xu S.; Liu X. H.; He Q.; Song L. J.; Ji H. B. ACS Catal. 2021, 11, 6810.
doi: 10.1021/acscatal.1c01350 pmid: 36378026 |
|
|
(r) Kim Y. L.; Park S.; Choi S. M.; Park J. U.; Kim J. H. Org. Lett. 2021, 23, 6674.
doi: 10.1021/acs.orglett.1c02219 pmid: 36378026 |
|
|
(s) Zhao X.; Yang F.; Zou S. Y.; Zhou Q. Q.; Chen Z. S.; Ji K. G. ACS Catal. 2022, 12, 1732.
doi: 10.1021/acscatal.1c05439 pmid: 36378026 |
|
|
(t) Lu C. H.; Li X. H.; Chang S. Q.; Zhang Y. Q.; Xing D. H.; Wang S.; Lin Y. P.; Jiang H. F.; Huang L. B. Org. Chem. Front. 2022, 9, 2382.
doi: 10.1039/D2QO00152G pmid: 36378026 |
|
|
(u) Mao R. Z.; Bera S.; Turla A. C.; Hu X. L. J. Am. Chem. Soc. 2021, 143, 14667.
doi: 10.1021/jacs.1c05874 pmid: 36378026 |
|
|
(v) Pal S.; Cotard M.; Gérardin B.; Hoarau C.; Schneider C. Org. Lett. 2021, 23, 3130.
doi: 10.1021/acs.orglett.1c00812 pmid: 36378026 |
|
|
(w) Dong Y. X.; Breit B. Org. Lett. 2021, 23, 6765.
doi: 10.1021/acs.orglett.1c02346 pmid: 36378026 |
|
|
(x) Ma J.-L.; Zhou X.-M.; Guo P.-H.; Cheng H.-C.; Ji H.-B. Chin. J. Chem. 2022, 40, 1204.
doi: 10.1002/cjoc.v40.10 pmid: 36378026 |
|
|
(y) Song Q. H.; Zhao H.; Sun Y. P.; Jiang H. F.; Zhang M. Chin. J. Chem. 2022, 40, 371.
doi: 10.1002/cjoc.v40.3 pmid: 36378026 |
|
|
(z) Tang K. H. N.; Uchida K.; Nishihara K.; Ito M.; Shibata T. Org. Lett. 2022, 24, 1313.
doi: 10.1021/acs.orglett.1c04321 pmid: 36378026 |
|
|
(aa) Tanaka K.; Hattori H.; Yabe R.; Nishimura T. Chem. Commun. 2022, 58, 5371.
doi: 10.1039/D2CC01275H pmid: 36378026 |
|
|
(ab) Shibata T.; Kojima M.; Onoda S.; Ito M. Org. Lett. 2021, 23, 8158.
doi: 10.1021/acs.orglett.1c02823 pmid: 36378026 |
|
|
(ac) Hoque M. E.; Bisht R.; Haldar C.; Chattopadhyay B. J. Am. Chem. Soc. 2017, 139, 7745.
doi: 10.1021/jacs.7b04490 pmid: 36378026 |
|
| [2] |
(a) Wu Y. T.; Pi C.; Wu Y. J.; Cui X. L. Chem. Soc. Rev. 2021, 50, 3677.
doi: 10.1039/D0CS00966K pmid: 35541005 |
|
(b) Yang K.; Song M. J.; Liu H.; Ge H. B. Chem. Sci. 2020, 11, 12616.
doi: 10.1039/d0sc03052j pmid: 35541005 |
|
|
(c) Abrams D. J.; Provencher P. A.; Sorensen E. J. Chem. Soc. Rev. 2018, 47, 8925.
doi: 10.1039/C8CS00716K pmid: 35541005 |
|
|
(d) Bhattacharya T.; Pimparkara S.; Maiti D. RSC Adv. 2018, 8, 19456.
doi: 10.1039/c8ra03230k pmid: 35541005 |
|
|
(e) Qin Y.; Zhu L. H.; Luo S. Z. Chem. Rev. 2017, 117, 9433.
doi: 10.1021/acs.chemrev.6b00657 pmid: 35541005 |
|
| [3] |
Zhu R. Y.; Farmer M. E.; Chen Y. Q.; Yu J. Q. Angew. Chem., Int. Ed. 2016, 55, 10578.
doi: 10.1002/anie.v55.36 |
| [4] |
(a) Li H. L.; Yang M.; Jin L. Y.; Yang Y. F.; She Y. B. J. Org. Chem. 2021, 86, 13475.
doi: 10.1021/acs.joc.1c01556 pmid: 28985082 |
|
(b) Yang S.; Wang F.; Wu Y. Q.; Hua W. K.; Zhang F. Z. Org. Lett. 2018, 20, 1491.
doi: 10.1021/acs.orglett.8b00071 pmid: 28985082 |
|
|
(c) Yu J. L.; Zhang S. Q.; Hong X. J. Am. Chem. Soc. 2017, 139, 7224.
doi: 10.1021/jacs.7b00714 pmid: 28985082 |
|
|
(d) Mandal A.; Sahoo H.; Dana S.; Baidya M. Org. Lett. 2017, 19, 4138.
doi: 10.1021/acs.orglett.7b01964 pmid: 28985082 |
|
|
(e) Bettadapur K. R.; Lanke V.; Prabhu K. R. Chem. Commun. 2017, 53, 6251.
doi: 10.1039/C7CC02392H pmid: 28985082 |
|
|
(f) Chen S. Q.; Li X. R.; Li C.-J.; Fan J.; Liu Z.-W.; Shi X.-Y. Org. Lett. 2020, 22, 1259.
doi: 10.1021/acs.orglett.9b04433 pmid: 28985082 |
|
|
(g) Srinivas K.; Siddiqui S. K.; Mudaliar J. K.; Ramana C. V. J. Org. Chem. 2019, 84, 5056.
doi: 10.1021/acs.joc.8b03267 pmid: 28985082 |
|
|
(h) Luo J. F.; Preciado S.; Araromi S. O.; Larrosa I. Chem.-Asian J. 2016, 11, 347.
doi: 10.1002/asia.v11.3 pmid: 28985082 |
|
|
(i) Liu X. Y.; Li X. Y.; Liu H.; Guo Q.; Lan J. B.; Wang R. L.; You J. S. Org. Lett. 2015, 17, 2936.
doi: 10.1021/acs.orglett.5b01171 pmid: 28985082 |
|
|
(j) Wang Y. W.; Li B.; Wang B. Q. Org. Lett. 2020, 22, 83.
doi: 10.1021/acs.orglett.9b03969 pmid: 28985082 |
|
|
(k) Barday M.; Janot C.; Halcovitch N. R.; Muir J.; Aïssa C. Angew. Chem., Int. Ed. 2017, 56, 13117.
doi: 10.1002/anie.v56.42 pmid: 28985082 |
|
|
(l) Shankar M.; Guntreddi T.; Ramesh E.; Sahoo A. K. Org. Lett. 2017, 19, 5665.
doi: 10.1021/acs.orglett.7b02824 pmid: 28985082 |
|
| [5] |
Gao R. Y.; Wang F.; Geng X.; Li C.-Y.; Wang L. Org. Lett. 2022, 24, 7118.
doi: 10.1021/acs.orglett.2c02703 |
| [6] |
(a) Zhang J.; Jiang J. W.; Li Y. L.; Wan X. B. J. Org. Chem. 2013, 78, 11366.
doi: 10.1021/jo401915t pmid: 22148366 |
|
(b) Potturi H. K.; Gurung R. K.; Hou Y. Q. J. Org. Chem. 2012, 77, 626.
doi: 10.1021/jo202276x pmid: 22148366 |
|
| [7] |
Lee J.; Chen L.; West A. H.; Richter-Addo G. B. Chem. Rev. 2002, 102, 1019.
doi: 10.1021/cr0000731 |
| [8] |
De S. R. Asian J. Org. Chem. 2021, 10, 980.
doi: 10.1002/ajoc.v10.5 |
| [9] |
Liu B. Q.; Fan Y.; Gao Y.; Sun C.; Xu C.; Zhu J. J. Am. Chem. Soc. 2013, 135, 468.
doi: 10.1021/ja3099245 |
| [10] |
Chen J. S.; Chen P.; Song C.; Zhu J. Chem.-Eur. J. 2014, 20, 14245.
doi: 10.1002/chem.v20.44 |
| [11] |
(a) Singh S.; Prasad N. R.; Chufan E. E.; Patel B. A.; Wang Y. J.; Chen Z.-S.; Ambudkar S. V.; Talele T. T. J.Med. Chem. 2014, 57, 4058.
pmid: 18076142 |
|
(b) Lee J.; Kim S. J.; Choi H.; Kim Y. H.; Lim I. T.; Yang H. M.; Lee C. S.; Kang H. R.; Ahn S. K.; Moon S. K.; Kim D.-H.; Lee S.; Choi N. S.; Lee K. J. J. Med. Chem. 2010, 53, 6337.
doi: 10.1021/jm1002414 pmid: 18076142 |
|
|
(c) Deng Y.; Chin Y.-W.; Chai H.; Keller W. J.; Kinghorn A. D. J. Nat. Prod. 2007, 70, 2049.
pmid: 18076142 |
|
| [12] |
Zhang L.; Wang Z.; Guo P. Y.; Sun W.; Li Y. M.; Sun M.; Hua C. W. Tetrahedron Lett. 2016, 57, 2511.
doi: 10.1016/j.tetlet.2016.04.096 |
| [13] |
Wu Y. N.; Sun L.; Chen Y. Y.; Zhou Q.; Huang J.-W.; Miao H.; Luo H.-B. J. Org. Chem. 2016, 81, 1244.
doi: 10.1021/acs.joc.5b02535 |
| [14] |
(a) Tian M. M.; Yang X. F.; Zhang B.; Liu B. X.; Li X. W. Org. Chem. Front. 2018, 5, 3406.
doi: 10.1039/C8QO00947C |
|
(b) Osada S.; Sano S.; Ueyama M.; Chuman Y.; Kodama H.; Sakaguchi K. Bioorg. Med. Chem. 2010, 18, 605.
doi: 10.1016/j.bmc.2009.12.005 |
|
|
(c) Oishi S.; Kamitani H.; Kodera Y.; Watanabe K.; Kobayashi K.; Narumi T.; Tomita K.; Ohno H.; Naito T.; Kodama E.; Matsuokab M.; Fujii N. Org. Biomol. Chem. 2009, 7, 2872.
doi: 10.1039/b907983a |
|
|
(d) Yang L.; Ji W.-W.; Lin E.; Li J.-L.; Fan W.-X.; Li Q. J.; Wang H. G. Org. Lett. 2018, 20, 1924.
doi: 10.1021/acs.orglett.8b00471 |
|
| [15] |
Tian M. M.; Yang X. F.; Zhang B.; Liu B. X.; Li X. W. Org. Chem. Front. 2018, 5, 3406.
doi: 10.1039/C8QO00947C |
| [16] |
Liu Z. S.; Zeng H.; Zhang W. J.; Song C.; Yang F.; Liu Y.; Zhu J. Polymer 2019, 172, 152.
doi: 10.1016/j.polymer.2019.03.063 |
| [17] |
He S.; Tan G. Y.; Luo A. P.; You J. S. Chem. Commun. 2018, 54, 7794.
doi: 10.1039/C8CC04027C |
| [18] |
Wang P.-L.; Wang Y.; Li Y.; Wang X.-S. Chem.-Asian J. 2020, 15,197.
|
| [19] |
Ouyang W. S.; Liu B. R.; He Y.; Wen Y. M.; Gao Y.; Huo Y. P.; Chen Q.; Li X. W. Org. Chem. Front. 2022, 9, 2746.
doi: 10.1039/D2QO00389A |
| [20] |
Peng R.-J.; Chen L.; Zhang X.-J.; Yan M. Adv. Synth. Catal. 2022, 364, 3567.
doi: 10.1002/adsc.v364.20 |
| [21] |
(a) Yu Y.; Jiang Y. M.; Wu S. F.; Shi Z. J.; Wu J. N.; Yuan Y. F.; Ye K.Y. Chin. Chem. Lett. 2022, 33, 2009.
doi: 10.1016/j.cclet.2021.10.016 |
|
(b) Wu Z.-L.; Chen J.-Y.; Tian X.-Z.; Ouyang W.-T.; Zhang Z.-T.; He W.-M. Chin. Chem. Lett. 2022, 33, 1501.
doi: 10.1016/j.cclet.2021.08.071 |
|
|
(c) Jiang J.; Wang Z.; He W.-M. Chin. Chem. Lett. 2021, 32, 1591.
doi: 10.1016/j.cclet.2021.02.067 |
|
|
(d) Li Z. B.; Sun Q.; Qian P.; Hu K. F.; Zha Z. G.; Wang Z. Y. Chin. Chem. Lett. 2020, 31, 1855.
doi: 10.1016/j.cclet.2020.02.030 |
|
| [22] |
Cao Y. M.; Yuan Y.; Lin Y. P.; Jiang X. M.; Weng Y. Q.; Wang T. W.; Bu F. X.; Zeng L.; Lei A. W. Green Chem. 2020, 22, 1548.
doi: 10.1039/D0GC00289E |
| [23] |
Wang X. Y.; Wu S. H.; Zhong Y. J.; Wang Y. C.; Pan Y. M.; Tang H. T. Chin. Chem. Lett. 2023, 34, 107537.
doi: 10.1016/j.cclet.2022.05.051 |
| [24] |
(a) Gao T. T.; Sun P. P. J. Org. Chem. 2014, 79, 9888.
doi: 10.1021/jo501902d |
|
(b) Gao T. T.; Yuan Y.; Liu P.; Sun P. P. Chin. Sci. Bull. 2015, 60, 2942. (in Chinese)
doi: 10.1360/N972015-00159 |
|
|
(高婷婷, 袁瑶, 刘平, 孙培培, 科学通报, 2015, 60, 2942.)
|
|
| [25] |
Li D.-D.; Cao Y.-X.; Wang G.-W.; Org. Biomol. Chem. 2015, 13, 6958.
doi: 10.1039/C5OB00691K |
| [26] |
Chen Y. Y.; Zhang R.; Peng Q. J.; Xu L. T.; Pan X. H. Chem.-Asian J. 2017, 12, 2804.
|
| [27] |
Li L.; Wang G.-W. Tetrahedron 2018, 74, 4188.
doi: 10.1016/j.tet.2018.06.003 |
| [28] |
Jia X. D.; Yuan Y.; Sun Z.; Zhang S. W.; Zhang Y. X. Asian J. Org. Chem. 2019, 8, 2205.
doi: 10.1002/ajoc.v8.12 |
| [29] |
(a) Dabiri M.; Lehi N. F.; Movahed S. K.; Khavasi H. R. Org. Biomol. Chem. 2017, 15, 6264.
doi: 10.1039/c7ob01534h pmid: 26931094 |
|
(b) Testa C.; Gigot Ê.; Genc S.; Decréau R.; Roger J.; Hierso J. C. Angew. Chem., Int. Ed. 2016, 55, 5555.
doi: 10.1002/anie.v55.18 pmid: 26931094 |
|
|
(c) Wang Y. X.; Li G.-X.; Yang G. H.; He G.; Chen G. Chem. Sci. 2016, 7, 2679.
doi: 10.1039/C5SC04169D pmid: 26931094 |
|
|
(d) Pawar A. B.; Lade D. M. Org. Biomol. Chem. 2016, 14, 3275.
doi: 10.1039/c5ob02640g pmid: 26931094 |
|
|
(e) Ding Q. P.; Zhou X. L.; Pu S. Z.; Cao B. P. Tetrahedron 2015, 71, 2376.
doi: 10.1016/j.tet.2015.03.004 pmid: 26931094 |
|
| [30] |
Peng Q. J.; Hu J.; Huo J. Y.; Yuan H. S.; Xu L. T.; Pan X. H. Org. Biomol. Chem. 2018, 16, 4471.
doi: 10.1039/C8OB00601F |
| [31] |
Liu B. Q.; Song C.; Sun C.; Zhou S. G.; Zhu J. J. Am. Chem. Soc. 2013, 135, 16625.
doi: 10.1021/ja408541c |
| [32] |
Wang C. M.; Huang Y. Org. Lett. 2013, 15, 5294.
doi: 10.1021/ol402523x |
| [33] |
Wang J.; Wang M. Y.; Chen K. H.; Zha S. K.; Song C.; Zhu J. Org. Lett. 2016, 18, 1178.
doi: 10.1021/acs.orglett.6b00310 pmid: 26909684 |
| [34] |
Liang Y. J.; Jiao N. Angew. Chem., Int. Ed. 2016, 55, 4035.
doi: 10.1002/anie.v55.12 |
| [35] |
Chen Y. Y.; Zhang R.; Peng Q. J.; Xu L. T.; Pan X. H. Chem.-Asian J. 2017, 12, 2804.
|
| [36] |
Hu X. W.; Chen X.; Shao Y. X.; Xie H. S.; Deng Y. F.; Ke Z. F.; Jiang H. F.; Zeng W. ACS Catal. 2018, 8, 1308.
doi: 10.1021/acscatal.7b03668 |
| [37] |
Wang H. N.; Li S. Q.; Wang B. Q.; Li B. Org. Chem. Front. 2018, 5, 88.
doi: 10.1039/C7QO00746A |
| [38] |
Song X.; Gao C.; Li B.; Zhang X. Y.; Fan X. S. J. Org. Chem. 2018, 83, 8509.
doi: 10.1021/acs.joc.8b01098 pmid: 29898599 |
| [39] |
Liu Y. F.; Tian Y.; Su K. X.; Wang P. G.; Guo X.; Chen B. H. Org. Chem. Front. 2019, 6, 3973.
doi: 10.1039/C9QO01250H |
| [40] |
Wu Y. T.; Pi C.; Cui X. L.; Wu Y. J. Org. Lett. 2020, 22, 361.
doi: 10.1021/acs.orglett.9b03768 |
| [41] |
Cui X.-F.; Huang G.-S. Org. Biomol. Chem. 2020, 18, 4014.
doi: 10.1039/D0OB00723D |
| [42] |
Peng R.-J.; Chen L.; Zhang X.-J.; Yan M. Adv. Syn. Catal. 2022, 364, 3567.
doi: 10.1002/adsc.v364.20 |
| [43] |
Li Z. Y.; Chen X. J.; Zhong H. L.; Lin Y. T.; Gao Y.; Liu Y.; Chen Q.; Huo Y. P.; Li X. W. Chem. Commun. 2022, 58, 13959.
doi: 10.1039/D2CC05514G |
| [44] |
Liu C. S.; Dai Q. Z.; Li Y. Q.; Huang C. H.; Guo L. J.; Yang Z. J. Org. Chem. 2023, doi:10.1021/acs.joc.3c00517.
|
| [45] |
Li C.; Yang Y. C.; Fang F. F.; Liu C. Y.; Li C. P.; Wang D. C.; Liu H. Chin. J. Chem. 2023, 41, 1957.
doi: 10.1002/cjoc.v41.16 |
| [46] |
Wu Y. N.; Feng L.-J.; Lu X.; Kwong F. Y.; Luo H.-B. Chem. Commun. 2014, 50, 15352.
doi: 10.1039/C4CC07440H |
| [47] |
Dong J. W.; Wu Z. J.; Liu Z. Y.; Liu P.; Sun P. P. J. Org. Chem. 2015, 80, 12588.
doi: 10.1021/acs.joc.5b01666 |
| [48] |
Ouyang W. S.; Liu B. R.; He Y.; Wen Y. M.; Gao Y.; Huo Y. P.; Chen Q.; Li X. W. Org. Chem. Front. 2022, 9, 2746.
doi: 10.1039/D2QO00389A |
| [49] |
(a) Gao R.-H.; Zuo L.-L.; Wang F.; Li C.-Y.; Jiang H.-J.; Li P.-H.; Wang L. Chin. J. Org. Chem. 2022, 42, 1883. (in Chinese)
doi: 10.6023/cjoc202203006 |
|
(高润烨, 左玲玲, 王芳, 李传莹, 蒋华江, 李品华, 王磊, 有机化学, 2022, 42, 1883.)
doi: 10.6023/cjoc202203006 |
|
|
(b) Yang S.; Li P.; Wang Z.; Wang L. Org. Lett. 2017, 19, 3386.
doi: 10.1021/acs.orglett.7b01230 |
|
|
(c) Ji W.; Li P.; Yang S.; Wang L. Chem. Commun. 2017, 53, 8482.
doi: 10.1039/C7CC03693K |
|
|
(d) Zhao L.; Li P.; Xie X.; Wang L. Org. Chem. Front. 2018, 5, 1689.
doi: 10.1039/C8QO00229K |
|
|
(e) Ren Y.; Meng L.-G.; Peng T.; Wang L. Org. Lett. 2018, 20, 4430.
doi: 10.1021/acs.orglett.8b01714 |
|
|
(f) Zhang Y.; Chen W.; Jia X.; Wang L.; Li P. Chem. Commun. 2019, 55, 2785.
doi: 10.1039/C8CC10235J |
|
|
(g) Zhao L.; Li P.; Xie X.; Wang L. Org. Chem. Front. 2019, 6, 87.
doi: 10.1039/C8QO01079J |
|
|
(h) Ye R.; Ruan H.; Xu H.; Li Z.; Meng L.-G.; Wang L. Org. Chem. Front. 2021, 8, 5345.
doi: 10.1039/D1QO01082D |
|
|
(i) Ruan H.; Meng L.-G.; Zhu L.; Wang L. Adv. Synth. Catal. 2019, 361, 3217.
doi: 10.1002/adsc.v361.13 |
|
|
(j) Xu N.; Zhang Y.; Chen W.; Li P.; Wang L. Adv. Synth. Catal. 2018, 360, 1199.
doi: 10.1002/adsc.v360.6 |
|
|
(k) Mei Y.; Zhao L.; Liu Q.; Ruan S.; Wang L.; Li P. Green Chem. 2020, 22, 2270.
doi: 10.1039/D0GC00009D |
|
| [50] |
(a) Huo J.; Geng X.; Li W.; Zhang P.; Wang L. Org. Lett. 2023, 25, 512.
doi: 10.1021/acs.orglett.2c04227 |
|
(b) Wan Q.; Hou Z.-W.; Zhao X.-R.; Xie X.; Wang L. Org. Lett. 2023, 25, 1008.
doi: 10.1021/acs.orglett.3c00144 |
|
|
(c) Wang Z.; Wang L.; Wang Z.; Li P.; Zhang Y. Chin. Chem. Lett. 2021, 32, 429.
doi: 10.1016/j.cclet.2020.02.022 |
|
|
(d) Lv Y.; Hou Z.-W.; Li P.; Wang L. Org. Chem. Front. 2023, 10, 990.
doi: 10.1039/D2QO01425D |
|
|
(e) Pan S.; Song M.; Zuo L.; Geng X.; Wang L. J. Org. Chem. 2023, 88, 5586.
doi: 10.1021/acs.joc.3c00093 |
|
|
(f) Fang J.; Pan Z.; Liu T.; Rao Y.; Jiang H.; Ma Y. Org. Biomol. Chem. 2023, 21, 2355.
doi: 10.1039/D3OB00083D |
|
|
(g) Geng M.; Kuang J.; Miao M.; Ma Y. Org. Biomol. Chem. 2023, 21, 3101.
doi: 10.1039/D3OB00296A |
|
|
(h) Zhao P.; Wang L.; Guo X.; Chen J.; Wang Y.; Ma Y. Org. Lett. 2023, 25, 3314.
doi: 10.1021/acs.orglett.3c01145 |
|
|
(i) Zhang Z.; Hou Z.-W.; Chen H.; Li P.; Wang L. Green Chem. 2023, 25, 3543.
doi: 10.1039/D3GC00728F |
| [1] | 付雅彤, 孙超凡, 张丹, 金成国, 陆居有. 巢式-碳硼烷硼氢键官能化反应研究进展[J]. 有机化学, 2024, 44(2): 438-447. |
| [2] | 高晓阳, 翟锐锐, 陈训, 王烁今. 碳酸亚乙烯酯参与C—H键活化反应的研究进展[J]. 有机化学, 2023, 43(9): 3119-3134. |
| [3] | 董思凡, 李昊龙, 秦源, 范士明, 刘守信. 氨基酸作为瞬态导向基在碳氢键活化反应中的研究进展[J]. 有机化学, 2023, 43(7): 2351-2367. |
| [4] | 蒋旺, 史壮志. 芳烃间/对位选择性碳氢硼化反应研究进展[J]. 有机化学, 2023, 43(5): 1691-1705. |
| [5] | 吴孔川, 卢铠洪, 林建斌, 张慧君. 莱啉酰亚胺类化合物的邻位C—H键功能化研究进展[J]. 有机化学, 2023, 43(3): 1000-1011. |
| [6] | 孙美娇, 谭晶, 谭玉, 彭进松, 陈春霞. 钯催化3-(2-氨基嘧啶-4-基)吲哚2位C—H键芳基化反应的研究[J]. 有机化学, 2023, 43(11): 3945-3959. |
| [7] | 商铭洲, 张兰兰, 陈淼淼, 胡汪成, 何心伟, 陆红健. 铑催化的邻苯二甲酸酐与环状2-重氮-1,3-二酮和甲醇的串联C—H活化/跨环偶联/环化反应合成酯基官能化的并环异香豆素类化合物的研究[J]. 有机化学, 2022, 42(11): 3816-3823. |
| [8] | 付拯江, 曹晰晗, 尹健, 苟振宇, 伊学政, 蔡琥. 基于“一石二鸟”策略的羧基无痕导向其邻位C—H键官能团化反应[J]. 有机化学, 2022, 42(1): 67-74. |
| [9] | 徐曼, 夏远志. 铑(III)催化N-苯氧基乙酰胺与亚甲基氧杂环丁酮氧化还原中性的碳氢活化/环化反应的机理研究[J]. 有机化学, 2021, 41(8): 3272-3278. |
| [10] | 王威霖, 陈卫东, 骆钧飞, 解攀. N-氟代苯磺酰亚胺参与的过渡金属催化C—H氟化和胺化的研究进展[J]. 有机化学, 2021, 41(2): 543-552. |
| [11] | 欧阳班来, 郑燕霞, 夏克坚, 徐小玲, 王艺. 过渡金属催化的芳环磺酰胺化反应研究进展[J]. 有机化学, 2020, 40(5): 1188-1205. |
| [12] | 史传星, 冯陈国, 陈雅丽, 张曙盛, 林国强. 苯并富烯合成方法研究进展[J]. 有机化学, 2020, 40(4): 817-830. |
| [13] | 陈训, 王颖, 王烁今, 孔杜林, 文丽君, 翟锐锐, 赵珂, 白丽丽, 李友宾. 钌(II)-催化偶氮苯与乙醛酸乙酯的环化反应构建3-羧酸酯吲唑[J]. 有机化学, 2020, 40(3): 688-693. |
| [14] | 罗飞华. 过渡金属催化羧基导向C-H官能团化研究进展[J]. 有机化学, 2019, 39(11): 3084-3104. |
| [15] | 唐灏, 骆钧飞, 解攀. 脱氢偶联制备芳基醚方法的研究进展[J]. 有机化学, 2019, 39(10): 2735-2743. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
