有机化学 ›› 2024, Vol. 44 ›› Issue (7): 2077-2091.DOI: 10.6023/cjoc202401032 上一篇 下一篇
综述与进展
收稿日期:2024-01-26
修回日期:2024-03-05
发布日期:2024-04-10
基金资助:
Wenjuan Liua, Pinhong Chena,b( )
)
Received:2024-01-26
Revised:2024-03-05
Published:2024-04-10
Contact:
E-mail: Supported by:文章分享

官能化的碳环或杂环结构广泛存在于天然产物及其他生物活性分子中, 是一类极具合成价值的重要骨架. 而1,n-烯炔的环化反应是构建官能化的碳环或杂环化合物的最具原子经济性, 且高效的方法之一, 其中钯催化的1,6-烯炔的环化反应研究较为成熟, 取得了一系列重要进展. 聚焦于由炔烃亲核钯化启动的1,6-烯炔的环化反应, 按照炔烃的亲核钯化方式包括氢钯化、碳钯化、卤钯化和氧钯化等分类, 系统介绍了近二十年内钯催化1,6-烯炔的环化反应进展, 尤其是不对称催化反应, 探讨了反应的机理, 并展望了该领域今后发展的方向.
刘雯娟, 陈品红. 钯催化1,6-烯炔的环化反应研究[J]. 有机化学, 2024, 44(7): 2077-2091.
Wenjuan Liu, Pinhong Chen. Palladium-Catalyzed Cyclization of 1,6-Enynes[J]. Chinese Journal of Organic Chemistry, 2024, 44(7): 2077-2091.
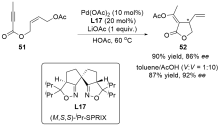
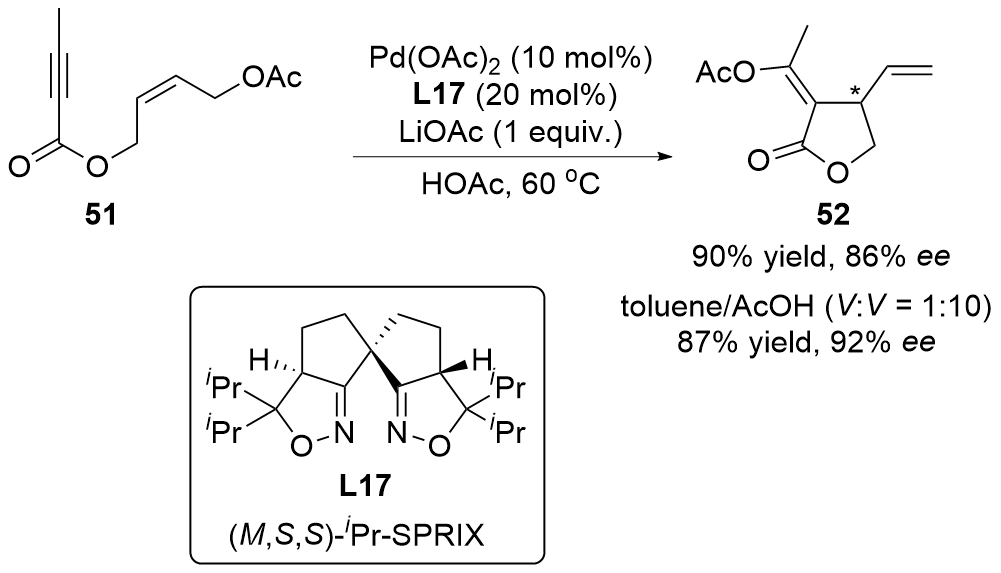
| [1] |
(a) Taylor, R. D.; MacCoss, M.; Lawson, A. D. G. J. Med. Chem. 2014, 57, 5845.
|
|
(b) Hammder, N.; Leth, L. A.; Stiller, J.; Jensen, M. E.; Jørgensen, K. A. Chem. Sci. 2016, 7, 3649.
|
|
| [2] |
For selected reviews, see: (a) Xu, C.-H.; Li, Y.; Li, J.-H.; Xiang, J.-N.; Deng, W. Sci. China Chem. 2019, 62, 1463.
|
|
(b) Chen, M.; Went, Y.; Lei, A. Prog. Chem. 2010, 22, 1341. (in Chinese)
|
|
|
(陈茂, 翁玥, 雷爱文, 化学进展, 2010, 22, 1341.)
|
|
|
(c) Michelet, V.; Toullec, P. Y.; Genêt, J.-P. Angew. Chem., Int. Ed. 2008, 47, 4268.
|
|
|
(d) Tong, X.; Zhang, Z. Chin. J. Org. Chem. 2004, 24, 591. (in Chinese)
|
|
|
(童晓峰, 张兆国, 有机化学, 2004, 24, 591.)
|
|
|
(e) Aubert, C.; Buisine, O.; Malacria, M. Chem. Rev. 2002, 102, 813.
|
|
|
(f) Trost, B. M. Acc. Chem. Res. 1990, 23, 34.
|
|
|
(g) Hu, L.-P.; Zhang, D.-R. Huang, X.-H.; Liu, F.-L; Li, X.; Teng, M.-Y.; Huang, G.-L. Chin. J. Chem. 2022, 40, 2756.
|
|
|
(h) Liu, J.-X.; Xu, S.-Q.; Han, Y.-P.; Liang, Y.-M. Adv. Synth. Catal. 2024, 366, 1220.
|
|
| [3] |
Trost, B. M.; Lautens, M. J. Am. Chem. Soc. 1985, 107, 1781.
|
| [4] |
Takacs, J. M.; Zhu, J.; Chandramouli, S. V. J. Am. Chem. Soc. 1992, 124, 773.
|
| [5] |
Fairlamb, I. J. S. Angew. Chem., Int. Ed. 2004, 43, 1048.
|
| [6] |
Lu, X.; Zhu, G.; Wang. Z. Synlett 1998, 115.
|
| [7] |
(a) Huang, X.; Nguyen, M. H.; Pu, L.; Zhang, L.; Chi, Y. R.; Wu, Y.-D.; Zhou, J. S. Angew. Chem., Int. Ed. 2020, 59, 10814.
|
|
(b) Li, Q.; Zhang, Y.; Liu, P.; Zhong, J.; Gong, B.; Yao, H.; Lin, A. Angew. Chem., Int. Ed. 2023, 62, e202211988.
|
|
| [8] |
Trost, B. M.; Romero, D. L.; Rise, F. J. Am. Chem. Soc. 1994, 116, 4268.
|
| [9] |
Trost, B. M.; Pedregal, C. J. Am. Chem. Soc. 1992, 114, 7292.
|
| [10] |
Oh, C. H.; Jung, H. H.; Kim, J. S.; Cho, S. W. Angew. Chem., Int. Ed. 2000, 39, 752.
pmid: 10760858 |
| [11] |
Hatano, M.; Terada, M.; Mikami, K. Angew. Chem., Int. Ed. 2001, 40, 249.
|
| [12] |
Hatano, M.; Mikami, K. J. Am. Chem. Soc. 2003, 125, 4704.
|
| [13] |
(a) Hatano, M.; Yamanaka, M.; Mikami, K. Eur. J. Org. Chem. 2003, 2552.
|
|
(b) Hatano, M.; Mikami, K. Org. Biomol. Chem. 2003, 1, 3871.
|
|
| [14] |
Quan, X.; Liu, J.; Rabten, W.; Diomedi, S.; Singh, T.; Andersson, P. G. Eur. J. Org. Chem. 2016, 3427.
|
| [15] |
Ren, X.; Tang, L.; Shen, C.; Li, H.; Wang, P.; Dong, K. Org. Lett. 2021, 23, 3561.
|
| [16] |
Marco-Martínez, J.; López-Carrillo, V.; Buñuel, E.; Simancas, R.; Cárdenas, D. J. J. Am. Chem. Soc. 2007, 129, 1874.
pmid: 17260998 |
| [17] |
Sun, D.; Zhou, B.; Liu, L.; Chen, X.; Hou, H.; Han, Y.; Yan, C.; Shi, Y.; Zhu, S. Org. Lett. 2023, 25, 4677.
|
| [18] |
Jia, X.; Petrone, D. A.; Lautens, M. Angew. Chem., Int. Ed. 2012, 51, 9870.
|
| [19] |
Petrone, D. A.; Franzoni, I.; Ye, J.; Rodríguez, J. F.; Poblador- Bahamonde, A. I.; Lautens, M. J. Am. Chem. Soc. 2017, 139, 3546.
doi: 10.1021/jacs.7b00482 pmid: 28195710 |
| [20] |
Wu, W.; Chen, T.; Chen, J.; Han, X. J. Org. Chem. 2017, 83, 1033.
|
| [21] |
Mikami, K.; Hatano, M. Proc. Natl. Acad. Sci. 2004, 101, 5767.
|
| [22] |
Dong, M.; Qi, L.; Qian, J.; Yu, S.; Tong, X. J. Am. Chem. Soc. 2023, 145, 1973.
|
| [23] |
(a) Kawasaki, S.; Satoh, T.; Miura, M.; Nomura, M. J. Org. Chem. 2003, 68, 6836.
pmid: 12919062 |
|
(b) Satoh, T.; Ogino, S.; Miura, M.; Nomura, M. Angew. Chem., Int. Ed. 2004, 43, 5063.
pmid: 12919062 |
|
|
(c) Oh, C. H.; Jung, H. H.; Kim, K. S.; Kim, N. Angew. Chem., Int. Ed. 2003, 42, 805.
pmid: 12919062 |
|
|
(d) For a review, see: Rajesh, M.; Kumar, G. R. Asian J. Org. Chem. 2023, 12, e202300106.
pmid: 12919062 |
|
| [24] |
Zhu, G.; Zhang, Z. Org. Lett. 2003, 5, 3645.
|
| [25] |
Meng, T. J.; Hu, Y.; Wang, S. J. Org. Chem. 2010, 75, 582.
|
| [26] |
Meng, T.; Hu, Y.; Sun, Y.; Zhu, T.; Wang, S. Tetrahedron 2010, 66, 8648.
|
| [27] |
Shen, K.; Han, X.; Lu, X. Org. Lett. 2012, 14, 1756.
doi: 10.1021/ol3003546 pmid: 22449236 |
| [28] |
Murthy, A. S.; Donikela, S.; Reddy, C. S.; Chegondi, R. J. Org. Chem. 2015, 80, 5566.
|
| [29] |
Shen, K.; Han, X.; Lu, X.; Hu, Z. Tetrahedron Lett. 2017, 58, 3768.
|
| [30] |
Jiang, M.; Jiang, T.; Bäckvall, J. E. Org. Lett. 2012, 14, 3538.
doi: 10.1021/ol301551x pmid: 22716930 |
| [31] |
Kaneda, K.; Uchiyama, T.; Fujiwara, Y.; Imanaka, T.; Teranish, S. J. Org. Chem. 1979, 44, 55.
|
| [32] |
(a) Ma, S.; Lu, X. J. Chem. Soc., Chem. Commun. 1990, 733.
|
|
(b) Ma, S.; Lu, X. J. Org. Chem. 1991, 56, 5120.
|
|
|
(c) Ma, S.; Zhu, G.; Lu, X. J. Org. Chem. 1993, 58, 3692.
|
|
|
(d) Ma, S.; Lu, X. J. Org. Chem. 1993, 58, 1245.
|
|
|
(e) Ji, J.; Lu, X. Tetrahedron 1994, 50, 9067.
|
|
|
(f) Ji, J. Zhang, C.; Lu, X. J. Org. Chem. 1995, 60, 1160.
|
|
|
(g) Wang, Z.; Lu, X. Tetrahedron Lett. 1997, 38, 5213.
|
|
| [33] |
Xie, X.; Lu, X.; Liu, Y.; Xu, W. J. Org. Chem. 2001, 66, 6545.
pmid: 11578203 |
| [34] |
Zhu, G.; Zhang, Z. J. Org. Chem. 2005, 70, 3339.
|
| [35] |
Yin, G.; Liu, G. Angew. Chem., Int. Ed. 2008, 47, 5442.
|
| [36] |
Takenaka, K.; Hashimoto, S.; Takizawa, S.; Sasai, H. Adv. Synth. Catal. 2011, 353, 1067.
|
| [37] |
Peng, H.; Liu, G. Org. Lett. 2011, 13, 772.
|
| [38] |
Tang, J.; Zhang, L.; Wu, W.; Yang, S.; Jiang, H. Chem.-Eur. J. 2022, 28, e202202528.
|
| [39] |
(a) Lu, X.; Zhu, G.; Ma, S. Tetrahedron Lett. 1992, 33, 7205.
|
|
(b) Wakabayashi, T.; Ishii, Y.; Murata, T.; Mizobe, Y.; Hidai, M. Tetrahedron Lett. 1995, 36, 5585.
|
|
| [40] |
Zhang, Q.; Lu, X. J. Am. Chem. Soc. 2000, 122, 7604.
|
| [41] |
Zhang, Q.; Lu, X.; Han, X. J. Org. Chem. 2001, 66, 7676.
pmid: 11701020 |
| [42] |
Song, J.; Shen, Q.; Xu, F.; Lu, X. Tetrahedron 2007, 63, 5148.
|
| [43] |
Xu, W.; Kong, A.; Lu, X. J. Org. Chem. 2006, 71, 3854.
|
| [44] |
Muthiah, C.; Arai, M. A.; Shinohara, T.; Arai, T.; Takizawa, S.; Sasai, H. Tetrahedron Lett. 2003, 44, 5201.
|
| [45] |
Zhao, L.; Lu, X.; Xu, W. J. Org. Chem. 2005, 70, 4059.
|
| [46] |
Bai, Y.-Q.; Wang, X.-W.; Wu, B.; Wang, X.-Q.; Liao, R.-Z.; Li, M.; Zhou, Y.-G. ACS Catal. 2023, 13, 9829.
|
| [47] |
Welbes, L. L.; Lyons, T. W.; Cychosz, K. A.; Sanford, M. S. J. Am. Chem. Soc. 2007, 129, 5836.
|
| [48] |
Tong, X.; Beller, M.; Tse, M. K. J. Am. Chem. Soc. 2007, 129, 4906.
|
| [49] |
Liu, H.; Yu, J.; Wang, L.; Tong, X. Tetrahedron Lett. 2008, 49, 6924.
|
| [50] |
Tsujihara, T.; Takenaka, K.; Onitsuka, K.; Hatanaka, M.; Sasai, H. J. Am. Chem. Soc. 2009, 131, 3452.
doi: 10.1021/ja809965e pmid: 19275254 |
| [51] |
Xu, T.; Wang, D.; Tong, X. Org. Lett. 2019, 21, 5368.
doi: 10.1021/acs.orglett.9b02096 pmid: 31247755 |
| [52] |
Vinoth, P.; Vivekanand, T.; Suryavanshi, P. A.; Menéndez, J. C.; Sasai, H.; Sridharan, V. Org. Biomol. Chem. 2015, 13, 5175.
|
| [53] |
Liu, B.; Song, R.-J.; Ouyang, X.-H.; Li, Y.; Hu, M.; Li, J.-H. Chem. Commun. 2015, 51, 12819.
|
| [54] |
Chen, J.; Han, X.; Lu, X. Angew. Chem., Int. Ed. 2017, 56, 14698.
|
| [55] |
Malik, G.; Swyka, R. A.; Tiwari, V. K.; Fei, X.; Applegate, G. A.; Berkowitz, D. B. Chem. Sci. 2017, 8, 8050.
doi: 10.1039/c7sc04083k pmid: 29568453 |
| [1] | 刘蒙金, 肖燕, 周锴, 李子成, 黄文才. 一锅法自偶联反应合成3,5-二苯基-1,2,4-噁二唑[J]. 有机化学, 2024, 44(7): 2251-2256. |
| [2] | 王文贵, 王守锋. 水溶液中的Minisci反应研究进展[J]. 有机化学, 2024, 44(7): 2136-2146. |
| [3] | 林雨, 付海峰, 曹华, 刘想. 碳酸亚乙烯酯: 有机合成化学中的多功能合成子[J]. 有机化学, 2024, 44(7): 2147-2173. |
| [4] | 张倩, 应垚璐, 张泓银, 徐林博, 林新奎, 黄晓雷. 钯催化SO2插入的炔丙基乙酸酯和碘代芳烃的还原偶联反应[J]. 有机化学, 2024, 44(6): 2033-2040. |
| [5] | 晏宇轩, 陆晚晴, 钱慧俊, 吕雷阳, 李志平. 钯催化偕二氟环丙烷开环与1,3-二羰基化合物的单/双氟烯丙基化反应[J]. 有机化学, 2024, 44(5): 1630-1640. |
| [6] | 罗东红, 李平, 陈志才, 杨佳怡, 孙梦凡, 陆居有. 钯催化邻-碳硼烷基吡啶卤化物交叉偶联合成邻-碳硼烷基联芳、氨基吡啶和炔基吡啶衍生物[J]. 有机化学, 2024, 44(5): 1568-1575. |
| [7] | 刘君君, 卢涛涛, 马平, 赵庆阳, 邝福儿. 钯催化的碳(sp3)-硅键转化实现碳(sp3)-碳(sp2)偶联制备三氟丙基(杂)芳烃[J]. 有机化学, 2024, 44(4): 1319-1326. |
| [8] | 吴际伟, 何俊, 王晶晶, 李丽霞, 徐采玉, 周洁, 李子荣, 许华建. 电化学氧化α-酮酸与邻氨基苄胺的脱羧环化反应[J]. 有机化学, 2024, 44(3): 972-980. |
| [9] | 刘杰, 韩峰, 李双艳, 陈天煜, 陈建辉, 徐清. 无过渡金属参与甲基杂环化合物与醇的选择性有氧烯基化反应[J]. 有机化学, 2024, 44(2): 573-583. |
| [10] | 孟宪强, 杨艺, 梁万洁, 王靖涛, 张荣葵, 刘会. 钯催化联烯胺区域选择性芳基酚氧化反应[J]. 有机化学, 2024, 44(1): 224-231. |
| [11] | 王兢睿, 冯永奎, 王能中, 黄年玉, 姚辉. 钯催化立体选择性合成硝基烷类β-碳糖苷[J]. 有机化学, 2023, 43(9): 3216-3225. |
| [12] | 王玉超, 刘晋彪, 何智涛. 钯催化共轭二烯的不对称氢官能团化[J]. 有机化学, 2023, 43(8): 2614-2627. |
| [13] | 张素珍, 张文文, 杨慧, 顾庆, 游书力. 铑催化2-烯基苯酚与炔烃的对映体选择性螺环化反应[J]. 有机化学, 2023, 43(8): 2926-2933. |
| [14] | 陈玉琢, 孙红梅, 王亮, 胡方芝, 李帅帅. 基于α-氢迁移策略构建杂环骨架的研究进展[J]. 有机化学, 2023, 43(7): 2323-2337. |
| [15] | 孙李星, 孙婷婷, 王海清, 吴淑芳, 王小烨, 刘天雅, 张宇辰. Lewis酸催化下3-烷基-2-吲哚烯与α,β-不饱和N-磺酰基亚胺的[2+4]环化反应[J]. 有机化学, 2023, 43(6): 2178-2188. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||