有机化学 ›› 2025, Vol. 45 ›› Issue (2): 602-619.DOI: 10.6023/cjoc202406002 上一篇 下一篇
综述与进展
收稿日期:2024-06-01
修回日期:2024-07-31
发布日期:2024-09-10
基金资助:Received:2024-06-01
Revised:2024-07-31
Published:2024-09-10
Contact:
*E-mail: leijiao@mail.tsinghua.edu.cn
Supported by:文章分享
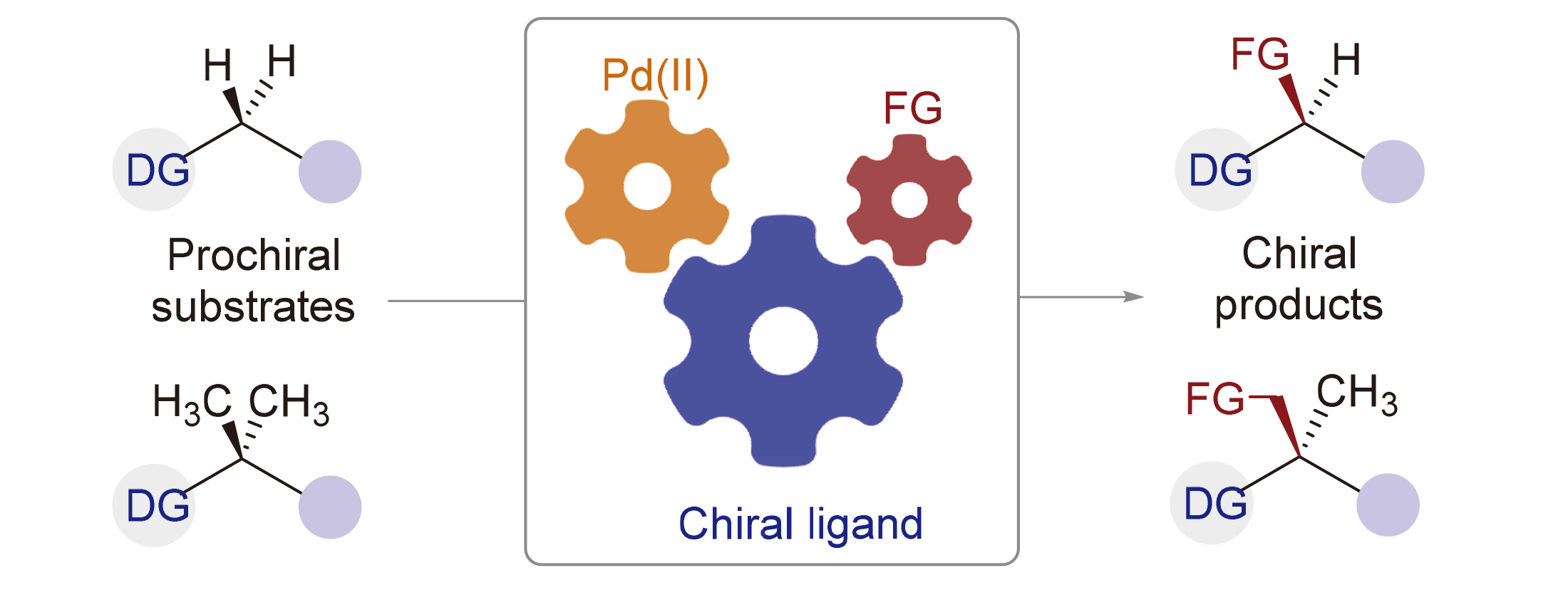
过渡金属催化的对映选择性C(sp3)—H键官能团化反应已成为构建手性分子的一种有效策略. 与其他过渡金属相比, 钯催化体系显示出良好的反应活性、多功能性和官能团耐受性, 是目前最常用的催化体系之一, 实现了一系列对映选择性C(sp3)—H键官能团化反应. 在这些反应中, 手性配体起到了举足轻重的作用, 是反应活性和立体选择性控制的关键因素. 聚焦于手性配体, 总结了近年来钯催化配位辅助对映选择性C(sp3)—H键官能团化反应的最新进展, 并着重介绍了手性配体的设计理念、反应机制和立体控制模型.
袁晨晖, 焦雷. 手性配体在钯催化配位辅助对映选择性C(sp3)—H键官能团化反应中的应用[J]. 有机化学, 2025, 45(2): 602-619.
Chen-Hui Yuan, Lei Jiao. Chiral Ligands for Palladium-Catalyzed Coordination-Assisted Enantioselective C(sp3)—H Functionalization Reactions[J]. Chinese Journal of Organic Chemistry, 2025, 45(2): 602-619.
| [1] |
(a) Guillemard, L.; Kaplaneris, N.; Ackermann, L.; Johansson, M. J. Nat. Rev. Chem. 2021, 5, 522.
doi: 10.1038/s41570-021-00300-6 pmid: 37117588 |
|
(b) Lam, N. Y. S.; Wu, K.; Yu, J.-Q. Angew. Chem., Int. Ed. 2021, 60, 15767.
pmid: 37117588 |
|
|
(c) Sinha, S. K.; Ghosh, P.; Jain, S.; Maiti, S.; Al-Thabati, S. A.; Alshehri, A. A.; Mokhtar, M.; Maiti, D. Chem. Soc. Rev. 2023, 52, 7461.
pmid: 37117588 |
|
| [2] |
(a) Achar, T. K.; Maiti, S.; Jana, S.; Maiti, D. ACS Catal. 2020, 10, 13748.
|
|
(b) Liu, B.; Romine, A. M.; Rubel, C. Z.; Engle, K. M.; Shi, B.-F. Chem. Rev. 2021, 121, 14957.
|
|
|
(c) Zhan, B.-B.; Jin, L.; Shi, B.-F. Trends Chem. 2022, 4, 220.
|
|
| [3] |
Siegbahn, P. E. M. J. Phy. Chem. 1995, 99, 12723.
|
| [4] |
Han, Y.-Q.; Shi, B.-F. Acta Chim. Sinica 2023, 81, 1522 (in Chinese).
|
|
(韩叶强, 史炳锋, 化学学报, 2023, 81, 1522.)
doi: 10.6023/A23070336 |
|
| [5] |
(a) Rouquet, G.; Chatani, N. Angew. Chem., Int. Ed. 2013, 52, 11726.
pmid: 30033454 |
|
(b) Sambiagio, C.; Schönbauer, D.; Blieck, R.; Dao-Huy, T.; Potot- schnig, G.; Schaaf, P.; Wiesinger, T.; Zia, M. F.; Wencel-Delord, J.; Besset, T.; Maes, B. U. W.; Schnürch, M. Chem. Soc. Rev. 2018, 47, 6603.
doi: 10.1039/c8cs00201k pmid: 30033454 |
|
| [6] |
(a) Wang, P.-S.; Gong, L.-Z. Chin. J. Chem. 2023, 41, 1841.
|
|
(b) Wang, P.-S.; Gong, L.-Z. Acc. Chem. Res. 2020, 53, 2841.
|
|
| [7] |
(a) Baudoin, O. Acc. Chem. Res. 2017, 50, 1114.
|
|
(b) Vyhivskyi, O.; Kudashev, A.; Miyakoshi, T.; Baudoin, O. Chem.-Eur. J. 2021, 27, 1231.
|
|
| [8] |
He, Y.-M.; Cheng, Y.-Z.; Duan, Y.; Zhang, Y.-D.; Fan, Q.-H.; You, S.-L.; Luo, S.; Zhu, S.-F.; Fu, X.-F.; Zhou, Q.-L. CCS Chem. 2023, 5, 2685.
|
| [9] |
(a) Shi, B.-F.; Maugel, N.; Zhang, Y.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2008, 47, 4882.
|
|
(b) Shao, Q.; Wu, K.; Zhuang, Z.; Qian, S.; Yu, J.-Q. Acc. Chem. Res. 2020, 53, 833.
|
|
| [10] |
Wasa, M.; Engle, K. M.; Lin, D. W.; Yoo, E. J.; Yu, J.-Q. J. Am. Chem. Soc. 2011, 133, 19598.
|
| [11] |
Chan, K. S. L.; Fu, H.-Y.; Yu, J.-Q. J. Am. Chem. Soc. 2015, 137, 2042.
|
| [12] |
Hu, L.; Shen, P.-X.; Shao, Q.; Hong, K.; Qiao, J. X.; Yu, J.-Q. Angew. Chem., Int. Ed. 2019, 58, 2134.
|
| [13] |
He, C.; Gaunt, M. J. Angew. Chem., Int. Ed. 2015, 54, 15840.
|
| [14] |
Rodrigalvarez, J.; Nappi, M.; Azuma, H.; Flodén, N. J.; Burns, M. E.; Gaunt, M. J. Nat. Chem. 2020, 12, 76.
doi: 10.1038/s41557-019-0393-8 pmid: 31863014 |
| [15] |
Rodrigalvarez, J.; Reeve, L. A.; Miró, J.; Gaunt, M. J. J. Am. Chem. Soc. 2022, 144, 3939.
|
| [16] |
Xiao, K.-J.; Lin, D. W.; Miura, M.; Zhu, R.-Y.; Gong, W.; Wasa, M.; Yu, J.-Q. J. Am. Chem. Soc. 2014, 136, 8138.
|
| [17] |
(a) Chen, G.; Gong, W.; Zhuang, Z.; Andrä, M. S.; Chen, Y.-Q.; Hong, X.; Yang, Y.-F.; Liu, T.; Houk, K. N.; Yu, J.-Q. Science 2016, 353, 1023.
|
|
(b) Andrä, M. S.; Schifferer, L.; Pollok, C. H.; Merten, C.; Gooßen, L. J.; Yu, J.-Q. Chem.-Eur. J. 2019, 25, 8503.
|
|
| [18] |
(a) He, J.; Shao, Q.; Wu, Q.; Yu, J.-Q. J. Am. Chem. Soc. 2017, 139, 3344.
|
|
(b) Wu, Q.-F.; Shen, P.-X.; He, J.; Wang, X.-B.; Zhang, F.; Shao, Q.; Zhu, R.-Y.; Mapelli, C.; Qiao, J. X.; Poss, M. A.; Yu, J.-Q. Science 2017, 355, 499.
|
|
|
(c) Shao, Q.; Wu, Q.-F.; He, J.; Yu, J.-Q. J. Am. Chem. Soc. 2018, 140, 5322.
|
|
| [19] |
Shen, P.-X.; Hu, L.; Shao, Q.; Hong, K.; Yu, J.-Q. J. Am. Chem. Soc. 2018, 140, 6545.
|
| [20] |
Jerhaoui, S.; Djukic, J.-P.; Wencel-Delord, J.; Colobert, F. ACS Catal. 2019, 9, 2532.
doi: 10.1021/acscatal.8b04946 |
| [21] |
Zhuang, Z.; Yu, J.-Q. J. Am. Chem. Soc. 2020, 142, 12015.
doi: 10.1021/jacs.0c04801 pmid: 32605367 |
| [22] |
Zhuang, Z.; Herron, A. N.; Yu, J.-Q. Angew. Chem., Int. Ed. 2021, 60, 16382.
|
| [23] |
Wang, P.; Farmer, M. E.; Huo, X.; Jain, P.; Shen, P.-X.; Ishoey, M.; Bradner, J. E.; Wisniewski, S. R.; Eastgate, M. D.; Yu, J.-Q. J. Am. Chem. Soc. 2016, 138, 9269.
|
| [24] |
Wang, Y.-J.; Yuan, C.-H.; Chu, D.-Z.; Jiao, L. Chem. Sci. 2020, 11, 11042.
doi: 10.1039/d0sc02246b pmid: 34094351 |
| [25] |
Yuan, C.-H.; Wang, X.-X.; Jiao, L. Angew. Chem., Int. Ed. 2023, 62, e202300854.
|
| [26] |
Yuan, C.-H.; Jiao, L. Org. Lett. 2024, 26, 29.
|
| [27] |
Zhang, T.; Zhang, Z.-Y.; Kang, G.; Sheng, T.; Yan, J.-L.; Yang, Y.-B.; Ouyang, Y.; Yu, J.-Q. Science 2024, 384, 793.
doi: 10.1126/science.ado1246 pmid: 38753778 |
| [28] |
(a) Maji, R.; Mallojjala, S. C.; Wheeler, S. E. Chem. Soc. Rev. 2018, 47, 1142.
|
|
(b) Li, X.; Song, Q. Chin. Chem. Lett. 2018, 29, 1181.
|
|
| [29] |
Connon, S. J. Angew. Chem., Int. Ed. 2006, 45, 3909.
|
| [30] |
(a) Wang, P.-S.; Gong, L.-Z. Synthesis 2021, 54, 4795.
|
|
(b) Sumit; Chandra, D.; Sharma, U. Chem. Commun. 2023, 59, 9288.
|
|
| [31] |
Yan, S.-B.; Zhang, S.; Duan, W.-L. Org. Lett. 2015, 17, 2458.
|
| [32] |
Wang, H.; Tong, H.-R.; He, G.; Chen, G. Angew. Chem., Int. Ed. 2016, 55, 15387.
|
| [33] |
Bay, K. L.; Yang, Y.-F.; Houk, K. N. J. Org. Chem. 2018, 83, 14786.
|
| [34] |
Zhang, Q.; Shi, B.-F. Acc. Chem. Res. 2021, 54, 2750.
|
| [35] |
Yan, S.-Y.; Han, Y.-Q.; Yao, Q.-J.; Nie, X.-L.; Liu, L.; Shi, B.-F. Angew. Chem., Int. Ed. 2018, 57, 9093.
|
| [36] |
Han, Y.-Q.; Zhang, Q.; Yang, X.; Jiang, M.-X.; Ding, Y.; Shi, B.-F. Org. Lett. 2021, 23, 97.
|
| [37] |
Jain, P.; Verma, P.; Xia, G.; Yu, J.-Q. Nat. Chem. 2017, 9, 140.
|
| [38] |
Jiang, H.-J.; Zhong, X.-M.; Liu, Z.-Y.; Geng, R.-L.; Li, Y.-Y.; Wu, Y.-D.; Zhang, X.; Gong, L.-Z. Angew. Chem., Int. Ed. 2020, 59, 12774.
|
| [39] |
Smalley, A. P.; Cuthbertson, J. D.; Gaunt, M. J. J. Am. Chem. Soc. 2017, 139, 1412.
doi: 10.1021/jacs.6b12234 pmid: 28064488 |
| [40] |
Han, Y.-Q.; Ding, Y.; Zhou, T.; Yan, S.-Y.; Song, H.; Shi, B.-F. J. Am. Chem. Soc. 2019, 141, 4558.
|
| [41] |
(a) Ding, Y.; Han, Y.-Q.; Wu, L.-S.; Zhou, T.; Yao, Q.-J.; Feng, Y.-L.; Li, Y.; Kong, K.-X.; Shi, B.-F. Angew. Chem., Int. Ed. 2020, 59, 14060.
pmid: 33683896 |
|
(b) Wu, L.-S.; Ding, Y.; Han, Y.-Q.; Shi, B.-F. Org. Lett. 2021, 23, 2048.
doi: 10.1021/acs.orglett.1c00204 pmid: 33683896 |
|
| [42] |
(a) Han, Y.-Q.; Yang, X.; Kong, K.-X.; Deng, Y.-T.; Wu, L.-S.; Ding, Y.; Shi, B.-F. Angew. Chem., Int. Ed. 2020, 59, 20455.
|
|
(b) Yang, X.; Jiang, M.-X.; Zhou, T.; Han, Y.-Q.; Xu, X.-T.; Zhang, K.; Shi, B.-F. Chem. Commun. 2021, 57, 5562.
|
|
| [43] |
Zhou, T.; Jiang, M.-X.; Yang, X.; Yue, Q.; Han, Y.-Q.; Ding, Y.; Shi, B.-F. Chin. J. Chem. 2020, 38, 242.
|
| [44] |
Jiang, M.-X.; Yang, X.; Han, Y.-Q.; Zhou, T.; Xu, X.-T.; Zhang, K.; Shi, B.-F. Org. Chem. Front. 2021, 8, 2903.
|
| [45] |
Tong, H.-R.; Zheng, W.; Lv, X.; He, G.; Liu, P.; Chen, G. ACS Catal. 2020, 10, 114.
|
| [46] |
Wang, C.-Y.; Zhou, T.; Shi, B.-F. ACS Catal. 2024, 14, 7213.
|
| [47] |
Tong, H.-R.; Zheng, S.; Li, X.; Deng, Z.; Wang, H.; He, G.; Peng, Q.; Chen, G. ACS Catal. 2018, 8, 11502.
|
| [48] |
Jiang, H.-J.; Zhong, X.-M.; Yu, J.; Zhang, Y.; Zhang, X.; Wu, Y.-D.; Gong, L.-Z. Angew. Chem., Int. Ed. 2019, 58, 1803.
|
| [49] |
Suseelan, A. S.; Dutta, A.; Lahiri, G. K.; Maiti, D. Trends Chem. 2021, 3, 188.
|
| [50] |
Yuan, C.-H.; Wang, X.-X.; Huang, K.; Jiao, L. Angew. Chem., Int. Ed. 2024, 63, e202405062.
|
| [1] | 姚团利, 王琳琳, 李涛. 钯催化多米诺Heck/分子间偶联反应合成3-烷基取代茚衍生物[J]. 有机化学, 2025, 45(4): 1342-1351. |
| [2] | 区洁晴, 屈培珍, 赵亮. 可见光介导下钯催化脂肪族α-溴代三氟甲基的脱溴还原反应[J]. 有机化学, 2025, 45(4): 1334-1341. |
| [3] | 白磊阳, 付蓓, 刘海平, 淳享, 姜雪峰. 白花前胡素E的全合成研究[J]. 有机化学, 2025, 45(3): 1009-1020. |
| [4] | 张朝威, 徐兵斌, 刘文龙, 赵敬, 段伟良. 钯催化不对称碳氢键活化合成平面手性二茂铁磺酰胺化合物[J]. 有机化学, 2025, 45(2): 707-716. |
| [5] | 令天鹏, 秦海涛, 刘峰. C(sp3)—H键对映选择性自由基反应的最新进展[J]. 有机化学, 2025, 45(2): 498-515. |
| [6] | 王淼, 黄雅豪, 胡鹏. 氢原子转移介导的烷烃C(sp3)—H选择性官能团化研究进展[J]. 有机化学, 2025, 45(2): 477-497. |
| [7] | 梅明顺, 张扬会. 钯催化惰性亚甲基C(sp3)—H键分子间官能团化反应[J]. 有机化学, 2025, 45(2): 620-640. |
| [8] | 刘雯娟, 陈品红. 钯催化1,6-烯炔的环化反应研究[J]. 有机化学, 2024, 44(7): 2077-2091. |
| [9] | 李非凡, 余康, 倪传志, 朱园园, 曾婕, 古双喜. 测定氨基酸浓度和对映体组成的手性荧光探针[J]. 有机化学, 2024, 44(6): 1862-1869. |
| [10] | 张倩, 应垚璐, 张泓银, 徐林博, 林新奎, 黄晓雷. 钯催化SO2插入的炔丙基乙酸酯和碘代芳烃的还原偶联反应[J]. 有机化学, 2024, 44(6): 2033-2040. |
| [11] | 晏宇轩, 陆晚晴, 钱慧俊, 吕雷阳, 李志平. 钯催化偕二氟环丙烷开环与1,3-二羰基化合物的单/双氟烯丙基化反应[J]. 有机化学, 2024, 44(5): 1630-1640. |
| [12] | 罗东红, 李平, 陈志才, 杨佳怡, 孙梦凡, 陆居有. 钯催化邻-碳硼烷基吡啶卤化物交叉偶联合成邻-碳硼烷基联芳、氨基吡啶和炔基吡啶衍生物[J]. 有机化学, 2024, 44(5): 1568-1575. |
| [13] | 刘君君, 卢涛涛, 马平, 赵庆阳, 邝福儿. 钯催化的碳(sp3)-硅键转化实现碳(sp3)-碳(sp2)偶联制备三氟丙基(杂)芳烃[J]. 有机化学, 2024, 44(4): 1319-1326. |
| [14] | 高淳, 刘欣, 王明慧, 刘淑贤, 朱婷婷, 张怡康, 郝二军, 杨启亮. 电化学不对称合成反应的研究进展[J]. 有机化学, 2024, 44(3): 673-727. |
| [15] | 王崔颖, 张颖, 郑家练, 袁佳, 孟思璇, 陈建, 余广鳌. 钯催化C—P键偶联反应高效构建手性联二萘基磷杂七元环化合物[J]. 有机化学, 2024, 44(12): 3771-3783. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
