有机化学 ›› 2025, Vol. 45 ›› Issue (2): 592-601.DOI: 10.6023/cjoc202406047 上一篇 下一篇
综述与进展
收稿日期:2024-06-29
修回日期:2024-07-26
发布日期:2024-09-10
基金资助:
Jingming Zhanga,b, Zhitao Hea,b,c( )
)
Received:2024-06-29
Revised:2024-07-26
Published:2024-09-10
Contact:
*E-mail: hezt@sioc.ac.cn
Supported by:文章分享
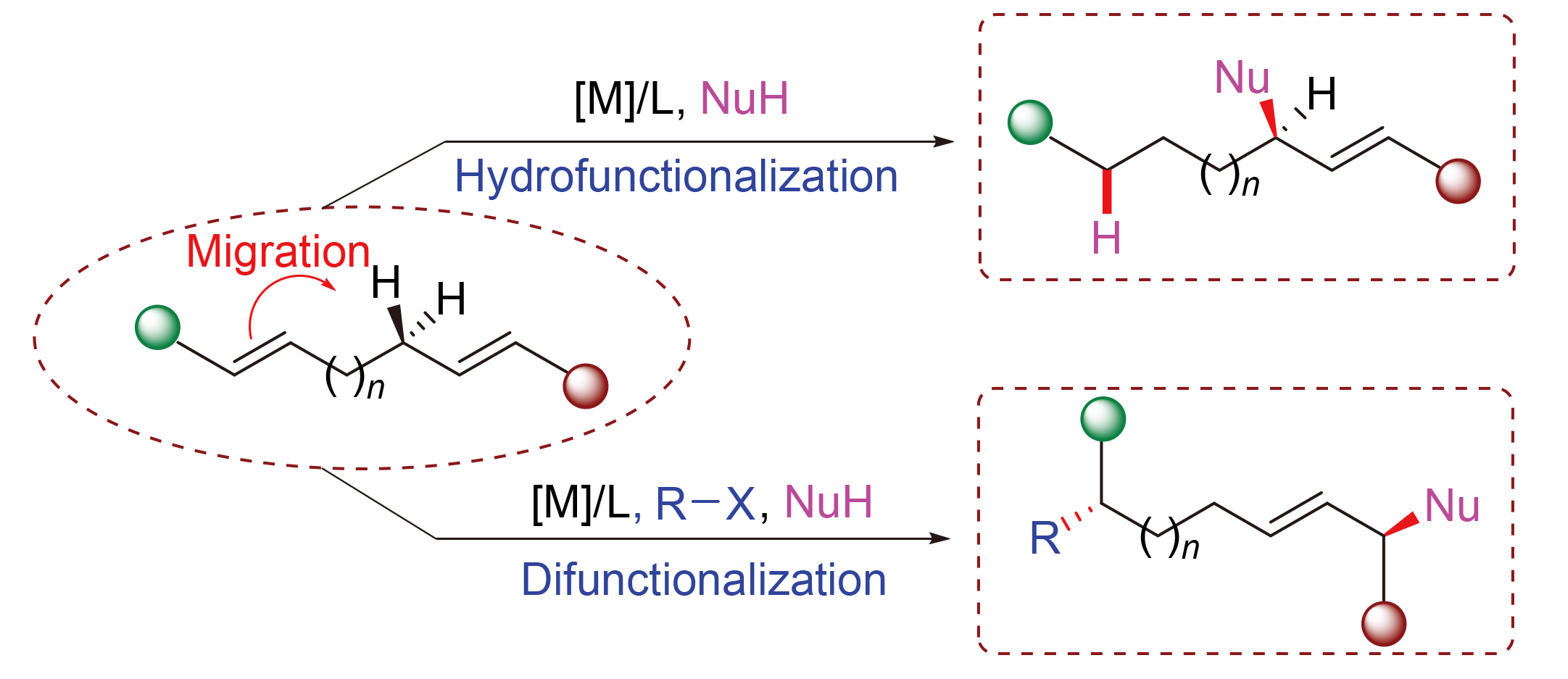
烯丙位碳氢键的不对称活化转化是具有重要价值和挑战性的一个研究方向. 不同于传统的直接脱氢策略, 过渡金属催化共轭二烯参与的迁移烯丙基取代反应是近年来发展起来的新的烯丙位碳氢键不对称活化转化的思路. 综述了该领域的发展历程和进展, 讨论了相关的机制过程, 并将其按照金属催化剂和产物类型进行了介绍.
张经明, 何智涛. 过渡金属催化远程二烯的不对称迁移烯丙位碳氢键官能团化[J]. 有机化学, 2025, 45(2): 592-601.
Jingming Zhang, Zhitao He. Transition Metal-Catalyzed Asymmetric Migratory Allylic C—H Functionalization of Remote Dienes[J]. Chinese Journal of Organic Chemistry, 2025, 45(2): 592-601.
| [1] |
For selected reviews and work on typical transition metal-catalyzed asymmetric allylic substitution, see: (a) Trost, B. M.; Vranken Van, D. L. Chem. Rev. 1996, 96, 395.
pmid: 33570909 |
|
(b) Trost, B. M.; Crawley, M. L. Chem. Rev. 2003, 103, 2921.
pmid: 33570909 |
|
|
(c) Lu, Z.; Ma, S. Angew. Chem., Int. Ed. 2008, 47, 258.
pmid: 33570909 |
|
|
(d) Trost, B. M. Tetrahedron 2015, 71, 5708.
pmid: 33570909 |
|
|
(e) Butt, N. A.; Zhang, W. Chem. Soc. Rev. 2015, 44, 7929.
pmid: 33570909 |
|
|
(f) Mohammadkhani, L.; Heravi, M. M. Chem. Rec. 2021, 21, 29.
pmid: 33570909 |
|
|
(g) Süsse, L.; Stoltz, B. M. Chem. Rev. 2021, 121, 4084.
doi: 10.1021/acs.chemrev.0c01115 pmid: 33570909 |
|
|
(h) Wang, R.-X.; Zhao, Q.-R.; Gu, Q.; You, S.-L. Acta Chim. Sinica 2023, 81, 431 (in Chinese).
pmid: 33570909 |
|
|
(王瑞祥, 赵庆如, 顾庆, 游书力, 化学学报, 2023, 81, 431.)
doi: 10.6023/A23030103 pmid: 33570909 |
|
| [2] |
For recent cases with C—C bond as leaving group, see: (a) Chen, Y.-W.; Qiu, Y.; Liu, Y.; Lin, G.-Q.; Hartwig, J. F. He, Z.-T. Nat. Synth. 2024, 3, 1011.
pmid: 39412244 |
|
(b) Liu, Y.; Chen, Y.-W.; Yang, Y.-X.; Hartwig, J. F.; He, Z.-T. J. Am. Chem. Soc. 2024, 146, 29857.
doi: 10.1021/jacs.4c11802 pmid: 39412244 |
|
| [3] |
For selected reviews, books and highlight on allylic C—H bond functionalization, see: (a) Jensen, T.; Fristrup, P. Chem.-Eur. J. 2009, 15, 9632.
|
|
(b) Liu, G.-S.; Wu, Y.-C. Activation, Springer, Berlin, Heidelberg, 2010, pp. 195-209.
|
|
|
(c) Liron, F.; Oble, J.; Lorion, M. M.; Poli, G. Eur. J. Org. Chem. 2014, 27, 5863.
|
|
|
(d) Tang, H.; Huo, X.; Meng, Q.; Zhang, W. Acta Chim. Sinica 2016, 74, 219 (in Chinese).
|
|
|
(汤淏溟, 霍小红, 孟庆华, 张万斌, 化学学报, 2016, 74, 219.)
doi: 10.6023/A16020078 |
|
|
(e) Wang, R.-H.; Luan, Y.-X.; Ye, M.-C. Chin. J. Chem. 2019, 37, 720.
|
|
|
(f) Wang, P.-S.; Gong, L.-Z. Acc. Chem. Res. 2020, 53, 2841.
|
|
|
(g) Yue, H.-F.; Zhu, C.; Huang, L.; Dewanjib, A.; Rueping, M. Chem. Commun. 2022, 58, 171.
|
|
| [4] |
(a) Li, J.-Y.; Zhang, Z.-H.; Wu, L.-Q.; Zhang, W.; Chen, P.-H.; Lin, Z.-Y.; Liu, G.-S. Nature 2019, 574, 516.
|
|
(b) Cheung, K. P. S.; Fang, J.; Mukherjee, K.; Mihranyan, A.; Gevorgyan, V. Science 2022, 378, 1207.
|
|
| [5] |
For selected reviews on metal-walking functionalization, see: (a) Vilches-Herrera, M.; Domke, L.; Bçrner, A. ACS Catal. 2014, 4, 1706.
|
|
(b) Larionov, E.; Li, H.; Mazet, C. Chem. Commun. 2014, 50, 9816.
|
|
|
(c) Vasseur, A.; Bruffaerts, J.; Marek, I. Nat. Chem. 2016, 8, 209.
|
|
|
(d) Sommer, H.; Juli-Hernndez, F.; Martin, R.; Marek, I. ACS Cent. Sci. 2018, 4, 153.
|
|
|
(e) Kochi, T.; Kanno, S.; Kakiuchi, F. Tetrahedron Lett. 2019, 60, 150938.
|
|
|
(f) Massad, I.; Marek, I. ACS Catal. 2020, 10, 5793.
|
|
|
(g) Li, Y.; Wu, D.; Cheng, H.-G.; Yin, G.-Y. Angew. Chem., Int. Ed. 2020, 59, 7990.
|
|
|
(h) Janssen-Mller, D.; Sahoo, B.; Sun, S.-Z.; Martin, R. Isr. J. Chem. 2020, 60, 195.
doi: 10.1002/ijch.201900072 |
|
| [6] |
For selected reviews on Pd-catalyzed enantioselective hydrofunctionalization of unsaturated bonds, see: (b) Haydl, A. M.; Breit, B.; Liang, T.; Krische, M. J. Angew. Chem., Int. Ed. 2017, 56, 11312.
pmid: 33301308 |
|
(b) Li, G.; Huo, X.; Jiang, X.; Zhang, W. Chem. Soc. Rev. 2020, 49, 2060.
pmid: 33301308 |
|
|
(c) Adamson, N. J.; Malcolmson, S. J. ACS Catal. 2020, 10, 1060.
pmid: 33301308 |
|
|
(d) Blieck, R.; Taillefer, M.; Monnier, F. Chem. Rev. 2020, 120, 13545.
doi: 10.1021/acs.chemrev.0c00803 pmid: 33301308 |
|
|
(e) Flaget, A.; Zhang, C.; Mazet, C. ACS Catal. 2022, 12, 15638.
pmid: 33301308 |
|
|
(f) Cera, G.; Maestri, G. ChemCatChem 2022, 14, e202200295.
pmid: 33301308 |
|
|
(g) Ma, C.; Chen, Y.-W.; He, Z.-T. Sci. Sin. Chim. 2023, 53, 474.
pmid: 33301308 |
|
|
(h) Wang, Y.-C.; Liu, J.-B.; He, Z.-T. Chin. J. Org. Chem. 2023, 43, 2614 (in Chinese).
pmid: 33301308 |
|
|
(王玉超, 刘晋彪, 何智涛, 有机化学, 2023, 43, 2614.)
doi: 10.6023/cjoc202302010 pmid: 33301308 |
|
| [7] |
For selected work on Pd-catalyzed enantioselective hydrofunctionalization of unsaturated bonds, see: (a) Löber, O.; Kawatsura, M.; Hartwig, J. F. J. Am. Chem. Soc. 2001, 123, 4366.
pmid: 32052974 |
|
(b) Leitner, A.; Larsen, J.; Steffens, C.; Hartwig, J. F. J. Org. Chem. 2004, 69, 7552.
pmid: 32052974 |
|
|
(c) Zhou, H.; Wang, Y.-N.; Zhang, L.; Cai, M.; Luo, S.-Z. J. Am. Chem. Soc. 2017, 139, 3631.
doi: 10.1021/jacs.7b00437 pmid: 32052974 |
|
|
(d) Adamson, N. J.; Hull, E.; Malcolmson, S. J. J. Am. Chem. Soc. 2017, 139, 7180.
doi: 10.1021/jacs.7b03480 pmid: 32052974 |
|
|
(e) Adamson, N. J.; E. Wilbur, K. C.; Malcolmson, S. J. J. Am. Chem. Soc. 2018, 140, 2761.
doi: 10.1021/jacs.7b13300 pmid: 32052974 |
|
|
(f) Nie, S.-Z.; Davison, R. T.; Dong, V. M. J. Am. Chem. Soc. 2018, 140, 16450.
pmid: 32052974 |
|
|
(g) Park, S.; Malcolmson, S. J. ACS Catal. 2018, 8, 8468.
pmid: 32052974 |
|
|
(h) Su, Y.-L.; Li, L.-L.; Zhou, X.-L.; Dai, Z.-Y.; Wang, P.-S.; Gong, L.-Z. Org. Lett. 2018, 20, 2403.
pmid: 32052974 |
|
|
(i) Zhang, Q.-L.; Yu, H.-M.; Shen, L.-L.; Tang, T.-H.; Dong, D.-F.; Chai, W.-W.; Zi, W.-W. J. Am. Chem. Soc. 2019, 141, 14554.
pmid: 32052974 |
|
|
(j) Park, S.; Adamson, N. J.; Malcolmson, S. J. Chem. Sci. 2019, 10, 5176.
pmid: 32052974 |
|
|
(k) Zhang, Z.; Xiao, F.; Wu, H.-M.; Dong, X.-Q.; Wang, C.-J. Org. Lett. 2020, 22, 569.
pmid: 32052974 |
|
|
(l) Onyeagusi, C. I.; Shao, X.; Malcolmson, S. J. Org. Lett. 2020, 22, 1681.
doi: 10.1021/acs.orglett.0c00342 pmid: 32052974 |
|
|
(m) Adamson, N. J.; Park, S.; Zhou, P.; Nguyen, A. L.; Malcolmson, S. J. Org. Lett. 2020, 22, 2032.
doi: 10.1021/acs.orglett.0c00412 pmid: 32052974 |
|
|
(n) Yang, H.; Xing, D. Chem. Commun. 2020, 56, 3721.
pmid: 32052974 |
|
|
(o) Zhang, Q.; Dong, D.; Zi, W. J. Am. Chem. Soc. 2020, 142, 15860.
pmid: 32052974 |
|
|
(p) Li, M.-M.; Cheng, L.; Xiao, L.-J.; Xie, J.-H.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2021, 60, 2948.
pmid: 32052974 |
|
|
(q) Yang, S.-Q.; Wang, Y.-F.; Zhao, W.-C.; Lin, G.-Q.; He, Z.-T. J. Am. Chem. Soc. 2021, 143, 7285.
pmid: 32052974 |
|
|
(r) Wang, H.; Zhang, R.; Zhang, Q.; Zi, W. J. Am. Chem. Soc. 2021, 143, 10948.
pmid: 32052974 |
|
|
(s) Jiu, A. Y.; Slocumb, H. S.; Yeung, C. S.; Yang, X.-H.; Dong, V. M. Angew. Chem., Int. Ed. 2021, 60, 19660.
pmid: 32052974 |
|
|
(t) Zhang, Q.; Zhu, M.; Zi, W. Chem 2022, 8, 2784.
pmid: 32052974 |
|
|
(u) Yang, S.-Q.; Han, A.-J.; Liu, Y.; Tang, X.-Y.; Lin, G.-Q.; He, Z.-T. J. Am. Chem. Soc. 2023, 145, 3915.
pmid: 32052974 |
|
|
(v) Tang, M.-Q.; Yang, Z.-J.; He, Z.-T. Nat. Commun. 2023, 14, 6303.
pmid: 32052974 |
|
|
(w) Han, A.-J.; Tan, Q.-T.; He, Z.-T. Org. Lett. 2024, 26, 89.
pmid: 32052974 |
|
|
(x) Yang, Z.-J.; He, Z.-T. Synthesis 2024, 56, 3412.
pmid: 32052974 |
|
|
(y) Zhang, J.-M.; Wang, Y.-C.; Chen, L.; Ma, C.; He, Z.-T. Chem.- Eur. J. 2024, 30, e202401350.
pmid: 32052974 |
|
|
(z) Xie, B.-Y.; He, Z.-T. ACS Catal. 2024, 14, 9742.
pmid: 32052974 |
|
| [8] |
Chen, Y.-W.; Liu, Y.; Lu, H.-Y.; Lin, G.-Q.; He, Z.-T. Nat. Commun. 2021, 12, 5626.
|
| [9] |
Liao, Q.-Y.; Ma, C.; Wang, Y.-C. Yang, S.-Q.; Ma, J.-S.; He, Z.-T. Chin. Chem. Lett. 2023, 34, 108371.
|
| [10] |
Wang, Y. -C, Xiao. Z.-X.; Wang, M.; Yang, S.-Q.; Liu, J.-B.; He, Z.-T. Angew. Chem., Int. Ed. 2023, 62, e202215568.
|
| [11] |
For selected examples on Pd-catalyzed asymmetric metal walking, see: (a) Werner, E.W.; Mei, T.-S.; Burckle, M.; Sigman, M. S. Science 2012, 338, 1455.
doi: 10.1126/science.1229208 pmid: 25723255 |
|
(b) Mei, T.-S.; Patel, H. H.; Sigman, M. S. Nature 2014, 508, 340.
pmid: 25723255 |
|
|
(c) Larionov, E.; Lin, L.; Guénée, L.; Mazet, C. J. Am. Chem. Soc. 2014, 136, 16882.
doi: 10.1021/ja508736u pmid: 25723255 |
|
|
(d) He, Y.; Yang, Z.; Thornbury, R. T.; Toste, F. D. J. Am. Chem. Soc. 2015, 137, 12207.
pmid: 25723255 |
|
|
(e) Nelson, H. M.; Williams, B. D.; Miró, J.; Toste, F. D. J. Am. Chem. Soc. 2015, 137, 3213.
doi: 10.1021/jacs.5b00344 pmid: 25723255 |
|
|
(f) Lin, L.; Romano, C.; Mazet, C. J. Am. Chem. Soc. 2016, 138, 10344.
pmid: 25723255 |
|
|
(g) Kou, X.; Shao, Q.; Ye, C.; Yang, G.; Zhang, W. J. Am. Chem. Soc. 2018, 140, 7587.
pmid: 25723255 |
|
|
(h) Liu, J.; Yuan, Q.; Toste, F. D.; Sigman, M. S. Nat. Chem. 2019, 11, 710.
pmid: 25723255 |
|
|
(i) Li, X.; Yang, T.; Li, J.; Li, X.; Chen, P.; Lin, Z.; Liu, G. Nat. Chem. 2023, 15, 862.
pmid: 25723255 |
|
| [12] |
Chen, X.-X.; Luo, H.; Chen, Y.-W.; Liu, Y.; He, Z.-T. Angew. Chem., Int. Ed. 2023, 62, e202307628.
|
| [13] |
Miao, H.-Z.; Liu, Y.; Chen, Y.-W.; Lu, H.-Y.; Li, J.; Lin, G.-Q.; He, Z.-T. Synlett 2023, 34, 451.
|
| [14] |
Li, G.-L.; Huo, X.-H.; Jiang, X.-Y.; Zhang, W.-B. Chem. Soc. Rev. 2020, 49, 2060.
|
| [15] |
Bender, D. D.; Stakem, F. G.; Heck, R. F. J. Org. Chem. 1982, 47, 1278.
|
| [16] |
Larock, R. C.; Lu, Y.-D.; Bain, A. C. J. Org. Chem. 1991, 56, 4589.
|
| [17] |
(a) Larock, R. C.; Berrios-Pefia, N. G.; Fried, C. A.; Yum, E. K.; Tu, C.; Leong, W. J. Org. Chem. 1993, 58, 4510.
|
|
(b) Larock, R. C.; Wang, Yao.; Lu, Y.-D.; Russell, C. E. J. Org. Chem. 1994, 59, 8107.
|
|
|
(c) Wang, Y.; Dong, X.-Y.; Larock, R. C. J. Org. Chem. 2003, 68, 3091.
|
|
| [18] |
Pang, H.-L.; Wu, D.; Cong, H.-J.; Yin, G.-Y. ACS Catal. 2019, 9, 8555.
|
| [19] |
Pang, H.-L.; Wu, D.; Yin, G.-Y. Chin. J. Org. Chem. 2021, 41, 849 (in Chinese).
|
|
(庞海亮, 吴冬, 阴国印, 有机化学, 2021, 41, 849.)
doi: 10.6023/cjoc202006022 |
|
| [20] |
Zhu, D.; Jiao, Z.; Chi, Y. R.; Goncalves, T. P.; Huang, K.-W.; Zhou, J. S. Angew. Chem., Int. Ed. 2020, 59, 5341.
|
| [21] |
Zhu, D.-Y.; Xu, W.-Q.; Pu, M.-P.; Wu, Y.-D.; Chi, Y. R.; Zhou, J. S. Org. Lett. 2021, 23, 7064.
|
| [22] |
Han, X.-J.; Larock, R. C. Synlett 1998, 7, 748.
|
| [23] |
Zhang, Y.; Shen, H.-C.; Li, Y.-Y.; Huang, Y.-S.; Han, Z.-Y.; Wu, X. Chem. Commun. 2019, 55, 3769.
|
| [24] |
Lux, M. C.; Boby, M. L.; Brooksc, J. L.; Tan, D. S. Chem. Commun. 2019, 55, 7013.
|
| [25] |
Yu, R.-R.; Rajasekar, S.; Fang, X.-J. Angew. Chem., Int. Ed. 2020, 59, 21436.
|
| [26] |
Cao, Y.-X.; Wodrich, M. D.; Cramer, D. Nat. Commun. 2023, 14, 7640.
|
| [27] |
Wang, X.; Miao, H.-Z.; Lin, G.-Q.; He, Z.-T. Angew. Chem., Int. Ed. 2023, 62, e202301556.
|
| [1] | 曾涛, 张曙盛, 付建国, 冯陈国. 防风中亥茅酚及亥茅酚苷的不对称合成[J]. 有机化学, 2024, 44(9): 2862-2868. |
| [2] | 梁蕾蕾, 姚家灿, 丁凡, 徐畅, 刘丹丹. 水鬼蕉碱Pancratistatin的合成方法研究进展[J]. 有机化学, 2024, 44(6): 1793-1810. |
| [3] | 陈远航, 何劲宇, 张博, 王延钊, 孔令轩, 钱伟烽, 王娜娜, 段闻喜, 欧阳妍妍, 朱翠菊, 徐浩. 不对称电化学有机合成[J]. 有机化学, 2024, 44(3): 748-779. |
| [4] | 于士航, 刘嘉威, 安碧玉, 边庆花, 王敏, 钟江春. 黑腹尼虎天牛接触性信息素的不对称合成[J]. 有机化学, 2024, 44(1): 301-308. |
| [5] | 王化坤, 任晓龙, 宣宜宁. 卤盐催化的α,β-环氧羧酸酯与异氰酸酯[3+2]环加成反应研究[J]. 有机化学, 2024, 44(1): 251-258. |
| [6] | 王玉超, 刘晋彪, 何智涛. 钯催化共轭二烯的不对称氢官能团化[J]. 有机化学, 2023, 43(8): 2614-2627. |
| [7] | 宋亭谕, 李冉, 黄利华, 贾世琨, 梅光建. N—N单键阻转异构体的催化不对称合成[J]. 有机化学, 2023, 43(6): 1977-1990. |
| [8] | 罗诚, 尹艳丽, 江智勇. P-手性膦氧化物的不对称合成研究进展[J]. 有机化学, 2023, 43(6): 1963-1976. |
| [9] | 蒙玲, 汪君. 硫代黄烷酮类衍生物的合成研究进展[J]. 有机化学, 2023, 43(3): 873-891. |
| [10] | 张怀远, 许诺, 唐蓉萍, 石星丽. 手性高价碘试剂诱导的不对称去芳构化反应研究进展[J]. 有机化学, 2023, 43(11): 3784-3805. |
| [11] | 匡鑫, 丁昌华, 吴奕晨, 王鹏. 手性烯丙基硅烷的催化对映选择性合成[J]. 有机化学, 2023, 43(10): 3367-3387. |
| [12] | 濮留洋, 李芷悦, 李利民, 马玉翠, 马民, 胡胜全, 吴正治. 秋水仙碱及其天然类似物(–)-N-乙酰秋水酚甲醚的不对称合成[J]. 有机化学, 2023, 43(1): 313-319. |
| [13] | 毛沅浩, 高延峰, 苗志伟. 过渡金属催化不对称环化反应合成七元环化合物研究进展[J]. 有机化学, 2022, 42(7): 1904-1924. |
| [14] | 姚婷, 李佳燕, 王佳明, 赵常贵. 氮杂环卡宾催化构筑含七元环结构的研究进展[J]. 有机化学, 2022, 42(4): 925-944. |
| [15] | 王立花, 公绪顺, 雷婷, 江世智. 黄烷酮的不对称合成研究进展[J]. 有机化学, 2022, 42(3): 758-769. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||