有机化学 ›› 2024, Vol. 44 ›› Issue (12): 3639-3646.DOI: 10.6023/cjoc202406052 上一篇 下一篇
综述与进展
杨露露, 易翠, 吴佳乐, 张宇轩, 白美琪, 李洋*( ), 徐四龙*(
), 徐四龙*( )
)
收稿日期:2024-06-30
修回日期:2024-08-27
发布日期:2024-09-10
基金资助:
Lulu Yang, Cui Yi, Jiale Wu, Yuxuan Zhang, Meiqi Bai, Yang Li*( ), Silong Xu*(
), Silong Xu*( )
)
Received:2024-06-30
Revised:2024-08-27
Published:2024-09-10
Contact:
*E-mail:Supported by:文章分享
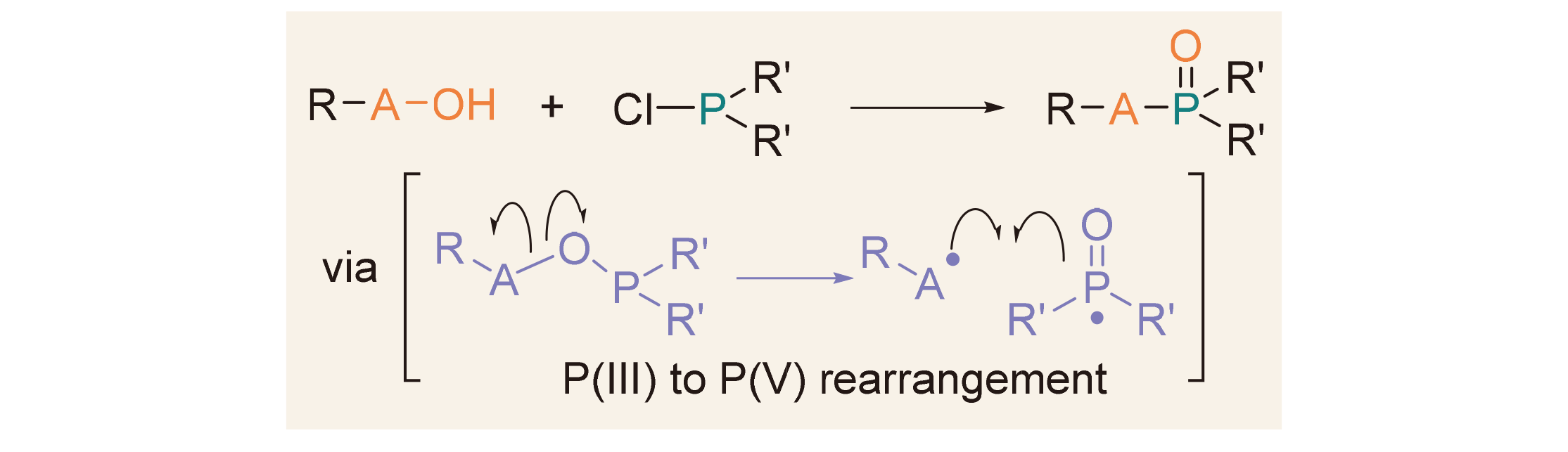
五价磷P(V)有机物广泛应用于合成、材料、生物、医药和农业等领域, 其合成方法研究一直吸引着有机化学家的兴趣. 从三价磷P(III)到五价磷P(V)的重排反应[P(III)→P(V)]是合成五价磷有机物的重要策略. 经典的Michaelis- Arbuzov反应通过亲核性亚磷酸酯[P(OR)3]与卤代烃发生P(III)→P(V)重排反应, 为膦酸酯的合成提供了重要方法. 与此相反, 亲电性氯化膦(ClPR1R2)与羟基化合物(RA—OH)作用可原位生成RA—O—PR1R2中间体, 继而发生A—O键断裂和A—P键形成, 从而实现P(III)→P(V)重排, 也为五价磷有机物的合成提供了有效方法. 这两类P(III)→P(V)重排反应都是在磷的强亲氧性驱动下实现的, 具有原子经济性高、底物简单易得等优点. 主要综述了氯化膦与不同类型羟基化合物(如醇、羧酸、肟和羟胺等)的P(III)→P(V)重排反应, 分别用于氧膦、膦酸酯、磷酰胺等五价磷化合物的合成.
杨露露, 易翠, 吴佳乐, 张宇轩, 白美琪, 李洋, 徐四龙. 氯化膦与羟基化合物参与的P(III)→P(V)重排反应研究进展[J]. 有机化学, 2024, 44(12): 3639-3646.
Lulu Yang, Cui Yi, Jiale Wu, Yuxuan Zhang, Meiqi Bai, Yang Li, Silong Xu. Progress in the P(III)→P(V) Rearrangement Reaction of Phosphine Chlorides and Hydroxyl Containing Compounds[J]. Chinese Journal of Organic Chemistry, 2024, 44(12): 3639-3646.
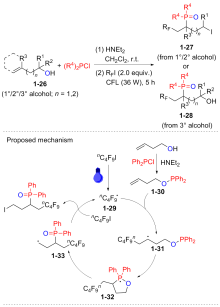
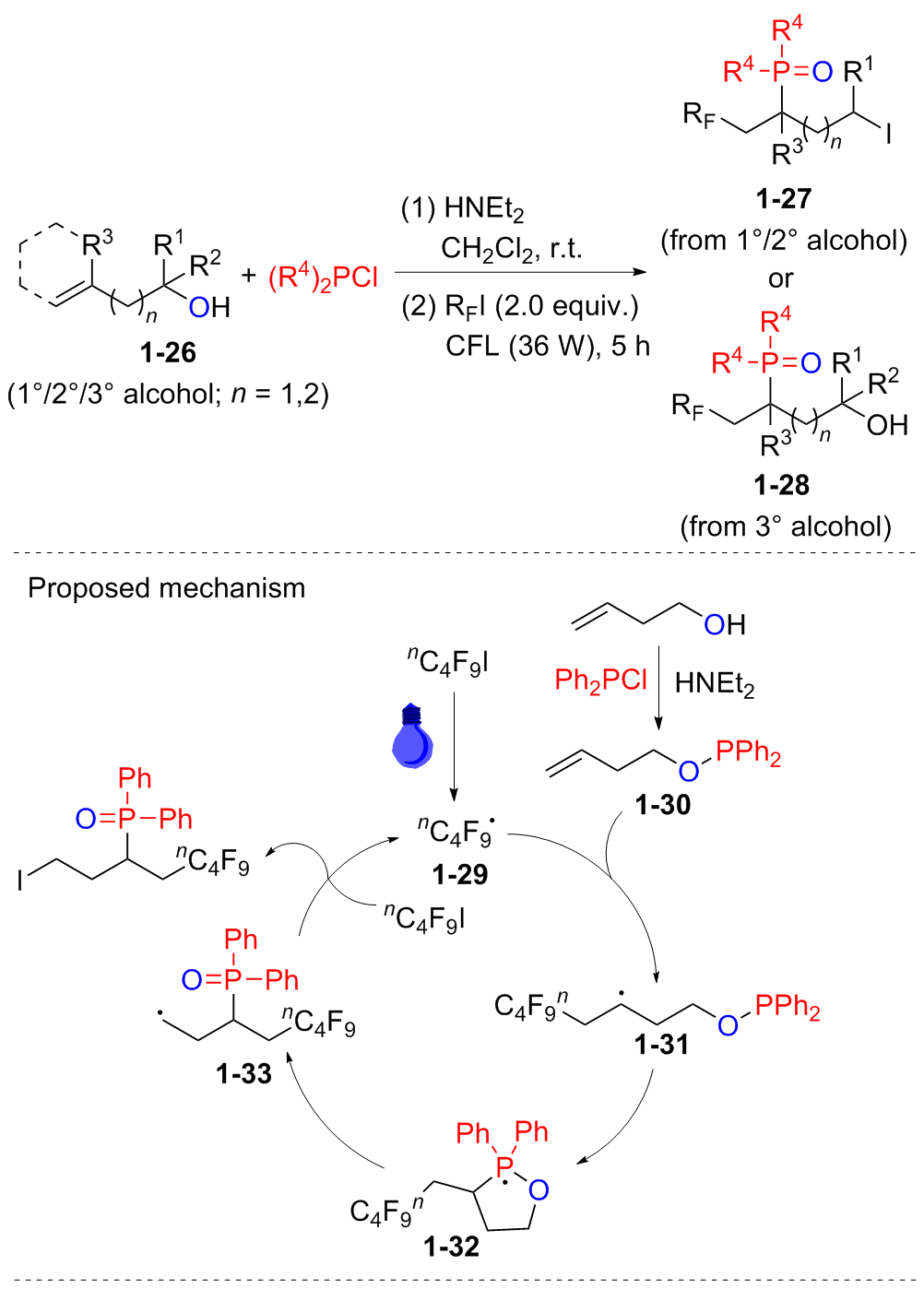
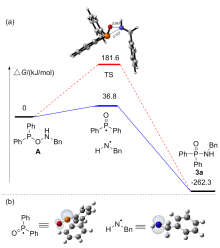

| [1] |
Perez H. F.; Etayo P.; Panossian A. Chem. Rev. 2011, 111, 2119.
|
| [2] |
van Leeuwen P. W. N. M.; Kamer P. C. J.; Claver C.; Pàmies O.; Diéguez M. Chem. Rev. 2011, 111, 2077.
doi: 10.1021/cr1002497 pmid: 21087011 |
| [3] |
Queffélec C.; Petit M.; Janvier P.; Knight D. A.; Bujoli B. Chem. Rev. 2012, 112, 3777.
doi: 10.1021/cr2004212 pmid: 22530923 |
| [4] |
Genet J.-P.; Ayad T.; Ratovelomanana-Vidal V. Chem. Rev. 2014, 114, 2824.
|
| [5] |
Pradere U.; Garnier-Amblard E. C.; Coats S. J.; Amblard F.; Schinazi R. F. Chem. Rev. 2014, 114, 9154.
doi: 10.1021/cr5002035 pmid: 25144792 |
| [6] |
Dutartre M.; Bayardon J.; Jugé S. Chem. Soc. Rev. 2016, 45, 5771.
pmid: 27479243 |
| [7] |
Eymery F.; Iorga B.; Savignac P. Tetrahedron 1999, 55, 13109.
|
| [8] |
Van der Jeught S.; Stevens C. V. Chem. Rev. 2009, 109, 2672.
doi: 10.1021/cr800315j pmid: 19449857 |
| [9] |
Shao C.; Xu W.; Li L.; Zhang X. Chin. J. Org. Chem. 2017, 37, 335 (in Chinese).
|
|
(邵长伟, 徐炜刚, 李亮, 张兴华, 有机化学, 2017, 37, 335.)
doi: 10.6023/cjoc201608030 |
|
| [10] |
Arbusow B. A. Pure Appl. Chem. 1964, 9, 307.
|
| [11] |
Bhattacharya A. K.; Thyagarajan G. Chem. Rev. 1981, 81, 415.
|
| [12] |
Xiong B.; Yuan M.; Shi C.; Zhu L.; Cao F.; Xu W.; Ren Y.; Liu Y.; Tang K. W. Top. Curr. Chem. 2024, 382, 10.
|
| [13] |
Chai L.; Wang J.; Yang J.; Yin J.; Zhang Z.; Cheng Y.; Zhu L.; Xue X. S.; Li C. CCS Chem. 2024, 6, 1312.
|
| [14] |
Lei Z.; Zhang W.; Wu J. ACS Catal. 2023, 13, 16105.
|
| [15] |
Yin J.; Lin X.; Chai L.; Wang C.; Zhu L.; Li C. Chem 2023, 9, 1945.
|
| [16] |
Zhang W.; Luo H. T.; Yang J. D. Chem 2023, 9, 2735.
|
| [17] |
Xu W.; Fan C.; Hu X.; Xu T. Angew. Chem., Int. Ed. 2024, 63, e202401575.
|
| [18] |
Rossi-Ashton J. A.; Clarke A. K.; Unsworth W. P.; Taylor R. J. K. ACS Catal. 2020, 10, 7250.
doi: 10.1021/acscatal.0c01923 pmid: 32905246 |
| [19] |
Kepp K. P. Inorg. Chem. 2016, 55, 9461.
|
| [20] |
Guo H.; Fan Y. C.; Sun Z.; Wu Y.; Kwon O. Chem. Rev. 2018, 118, 10049.
|
| [21] |
Wittig G.; Schöllkopf U. Chem. Ber. 2006, 87, 1318.
|
| [22] |
Wang X.; Yang F.; Xue Z.; Wang X. Chin. J. Org. Chem. 2015, 35, 29 (in Chinese).
|
|
(王小龙, 杨芳, 薛自燕, 王晓强, 有机化学, 2015, 35, 29.)
doi: 10.6023/cjoc201406003 |
|
| [23] |
Appel R. Angew. Chem., Int. Ed. 2003, 14, 801.
|
| [24] |
Liu Y.; Liu X.; Feng X. Chem. Sci. 2022, 13, 12290.
|
| [25] |
Boisselle A. P.; Meinhardt N. A. J. Org. Chem. 1962, 27, 1828.
|
| [26] |
Darcel C.; Bruneau C.; Dixneuf P. H. Synthesis 1996, 1996, 711.
|
| [27] |
Ismailov I. E.; Ivanov I. K.; Christov V. C. Molecules 2014, 19, 6309.
doi: 10.3390/molecules19056309 pmid: 24840901 |
| [28] |
Ismailov I. E.; Ivanov I. K.; Christov V. C. Molecules 2014, 19, 11056.
doi: 10.3390/molecules190811056 pmid: 25076142 |
| [29] |
Christov V. C.; Ismailov I. E.; Ivanov I. K. Molecules 2015, 20, 7263.
doi: 10.3390/molecules20047263 pmid: 25905604 |
| [30] |
Hasanov H. H.; Ivanov I. K.; Christov V. C. Heteroat. Chem 2017, 28, e21357.
|
| [31] |
Baumann M.; Baxendale I. R. J. Org. Chem. 2015, 80, 10806.
doi: 10.1021/acs.joc.5b01982 pmid: 26477591 |
| [32] |
Demay S.; Harms K.; Knochel P. Tetrahedron Lett. 1999, 40, 4981.
|
| [33] |
Liron F.; Knochel P. Chem. Commun. 2004, 304.
|
| [34] |
Masarwa A.; Stanger A.; Marek I. Angew. Chem., Int. Ed. 2007, 46, 8039.
|
| [35] |
Busacca C. A.; Qu B.; Farber E.; Haddad N.; Gret N.; Saha A. K.; Eriksson M. C.; Wu J.-P.; Fandrick K. R.; Han S. Org. Lett. 2013, 15, 1136.
doi: 10.1021/ol400310h pmid: 23427861 |
| [36] |
Shevchuk M.; Röschenthaler G.-V. Synthesis 2021, 54, 171.
|
| [37] |
Ma Y.; Chen F.; Bao J.; Wei H.; Shi M.; Wang F. Tetrahedron Lett. 2016, 57, 2465.
|
| [38] |
Xie D.-T.; Chen H.-L.; Wei D.; Wei B.-Y.; Li Z.-H.; Zhang J.-W.; Yu W.; Han B. Angew. Chem., Int. Ed. 2022, 61, e202203398.
|
| [39] |
Xie X.-X.; Yang Y.-C.; Dou B.-H.; Li Z.-F.; Li G. Coord. Chem. Rev. 2020, 403, 213100.
|
| [40] |
Li J.; Huang C.-Y.; Li C.-J. Angew. Chem., Int. Ed. 2022, 61, e202112770.
|
| [41] |
Sartori P.; Heinz Hochleitner R.; Hägele G. Naturforsch. Teil. B 1976, 31, 76.
|
| [42] |
Miller J. A.; Stewart D. Tetrahedron Lett. 1977, 18, 1065.
|
| [43] |
Kondoh A.; Ojima R.; Terada M. Org. Lett. 2021, 23, 7894.
|
| [44] |
Kaur R.; Singh R. P. J. Org. Chem. 2023, 88, 10325.
|
| [45] |
Kuroboshi M.; Ishihara T.; Ando T. J. Fluorine Chem. 1988, 39, 293.
|
| [46] |
Sartori P.; Mosler G. Phosphorus Sulfur Relat. Elem. 1980, 8, 115.
|
| [47] |
Bew S. P.; Brimage R. A.; Hughes D. L.; Legentil L.; Sharma S. V.; Wilson M. A. J. Org. Chem. 2007, 72, 2655.
|
| [48] |
Brown C.; Hudson R. F.; Maron A.; Record K. A. F. J. Chem. Soc., Chem. Commun. 1976, 663.
|
| [49] |
Yang L.; Wu J.; Li Y.; Tang Y.; Li J.; Xu S. Org. Lett. 2024, 26, 3208.
|
| [50] |
Xia P. J.; Ye Z. P.; Hu Y. Z.; Song D.; Xiang H. Y.; Chen X. Q.; Yang H. Org. Lett. 2019, 21, 2658.
|
| [51] |
Zhang J. J.; Duan X. H.; Wu Y.; Yang J. C.; Guo L. N. Chem. Sci. 2019, 10, 161.
|
| [52] |
Chen J.; He B.-Q.; Wang P.-Z.; Yu X.-Y.; Zhao Q.-Q.; Chen J.-R.; Xiao W.-J. Org. Lett. 2019, 21, 4359.
doi: 10.1021/acs.orglett.9b01529 pmid: 31141377 |
| [53] |
Davies J.; Morcillo S. P.; Douglas J. J.; Leonori D. Chem. Eur. J. 2018, 24, 12154.
|
| [54] |
Gao F.; Auclair K. Phosphorus, Sulfur Silicon Relat. Elem. 2006, 181, 159.
|
| [55] |
Banks M. R.; Hudson R. F. J. Chem. Soc., 1989, 463.
|
| [56] |
Cheng F.; Li D.; Li J.; Tang Y.; Wu Y.; Xu S. Org. Lett. 2023, 25, 2555.
doi: 10.1021/acs.orglett.3c00229 pmid: 36876752 |
| [1] | 方思强, 刘赞娇, 王天利. Atherton-Todd反应的研究进展[J]. 有机化学, 2023, 43(3): 1069-1083. |
| [2] | 郭泽, 吴迪, 王丽丽, 段征. BF3•Et2O促进的双烯酮-酚重排合成具有聚集诱导发光(AIE)效应的磷杂七元环化合物[J]. 有机化学, 2022, 42(8): 2481-2487. |
| [3] | 杨治芳, 程乙夫, 张蓓蓓, 董韵怡, 韩驰, 杜云飞. 高价碘试剂介导下的氧化重排反应[J]. 有机化学, 2022, 42(11): 3456-3505. |
| [4] | 兰新婵, 王丽丽, 段征, François Mathey. 磷杂Fries重排反应用于合成2-芘基膦化合物[J]. 有机化学, 2021, 41(3): 1153-1160. |
| [5] | 仝明慧, 张欣宇, 王也铭, 王自坤. 碘叶立德的化学反应研究进展[J]. 有机化学, 2021, 41(1): 126-143. |
| [6] | 韩满意, 潘虹, 姚紫云, 李琦. 四丁基溴化铵催化的布鲁克重排/烷基化反应[J]. 有机化学, 2020, 40(12): 4274-4283. |
| [7] | 王凯璇, 王兰芝. 非预期的重排反应及苯并噁唑类化合物的合成[J]. 有机化学, 2019, 39(4): 1147-1152. |
| [8] | 雷禄, 李承璟, 莫冬亮. 铜催化N-O键断裂策略研究进展[J]. 有机化学, 2019, 39(11): 2989-3012. |
| [9] | 刘双, 李玉明, 王典, 魏榕, 苗志伟. 手性诱导构建磷手性中心不对称合成有机磷功能化合物研究进展[J]. 有机化学, 2018, 38(2): 341-349. |
| [10] | 张珠, 徐希森, 李彦龙, 李家柱, 王进军. 焦脱镁叶绿酸与重氮烷的重排反应及其叶绿素衍生物的合成[J]. 有机化学, 2018, 38(11): 2993-3001. |
| [11] | 蔺松波, 何兴瑞, 孟金鹏, 顾海宁, 张培志, 吴军. 无过渡金属存在下由邻卤苯酚合成邻卤二芳胺[J]. 有机化学, 2017, 37(7): 1864-1869. |
| [12] | 杨佳, 肖晶, 周永波, 陈铁桥, 尹双凤, 韩立彪. 简单易得试剂与磷-氢化合物交叉偶联合成有机磷化合物[J]. 有机化学, 2017, 37(5): 1055-1068. |
| [13] | 叶美君, 张培志, 吴军. 2-嘧啶氧基-N-芳基苄胺类衍生物的酸催化Smiles重排反应条件的快速筛选和产物的高效制备[J]. 有机化学, 2016, 36(12): 2997-3002. |
| [14] | 张翔, 丛颖, 林光宇, 郭旭亮, 曹阳, 雷坤华, 杜云飞. 有机三价碘试剂在杂环化合物合成中的应用进展[J]. 有机化学, 2016, 36(11): 2513-2529. |
| [15] | 杨鑫, 孙中强, 邓云霞, 邵志宇. 藤黄酸重排反应产物研究[J]. 有机化学, 2015, 35(4): 917-921. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||