有机化学 ›› 2024, Vol. 44 ›› Issue (9): 2722-2731.DOI: 10.6023/cjoc20203013 上一篇 下一篇
综述与进展
曾玉, 黎忠昊, 邓思威, 陈祖佳, 陈璧瑜, 宇世伟*( ), 沈晴, 汪朝阳*(
), 沈晴, 汪朝阳*( )
)
收稿日期:2024-03-12
修回日期:2024-04-15
发布日期:2024-05-10
通讯作者:
宇世伟, 汪朝阳
基金资助:
Yu Zeng, Zhonghao Li, Siwei Deng, Zujia Chen, Biyu Chen, Shiwei Yu( ), Qing Shen, Zhaoyang Wang(
), Qing Shen, Zhaoyang Wang( )
)
Received:2024-03-12
Revised:2024-04-15
Published:2024-05-10
Contact:
Shiwei Yu, Zhaoyang Wang
Supported by:文章分享

氰基乙烯类是一类较易制备的π-π共轭化合物, 可通过Knoevenagel缩合反应、多组分反应、串联反应等绿色化方法获得. 氰基乙烯类中的特殊D-π-A类结构单元, 使其光学性质可应用于荧光探针、染料敏化电池中的敏化剂等领域. 同时, 氰基乙烯类化合物中β-位碳、氰基碳、γ-位碳电子云密度的不同影响着它们的化学反应性质, 使其可作为不同的合成子, 通过Michael加成型和环加成型等反应, 较温和地构建各种含氮化合物, 特别是环状化合物. 以合成氰基乙烯类化合物的类型为依据, 总结了其常见的制备策略; 按照在不同光学领域的应用, 介绍了氰基乙烯型荧光探针和电池敏化剂; 按照反应类型和产物结构, 综述了近年来其在有机合成领域的应用. 展望未来, 人们将会对其性能与应用有更多的了解, 设计更多性能优异的荧光探针分子, 同时深入发展氰基乙烯类化合物的有机合成应用.
曾玉, 黎忠昊, 邓思威, 陈祖佳, 陈璧瑜, 宇世伟, 沈晴, 汪朝阳. 氰基乙烯类化合物的制备与应用研究进展[J]. 有机化学, 2024, 44(9): 2722-2731.
Yu Zeng, Zhonghao Li, Siwei Deng, Zujia Chen, Biyu Chen, Shiwei Yu, Qing Shen, Zhaoyang Wang. Research Progress on Preparation and Application of Cyanoethylenes[J]. Chinese Journal of Organic Chemistry, 2024, 44(9): 2722-2731.
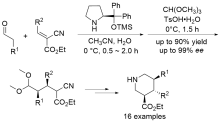
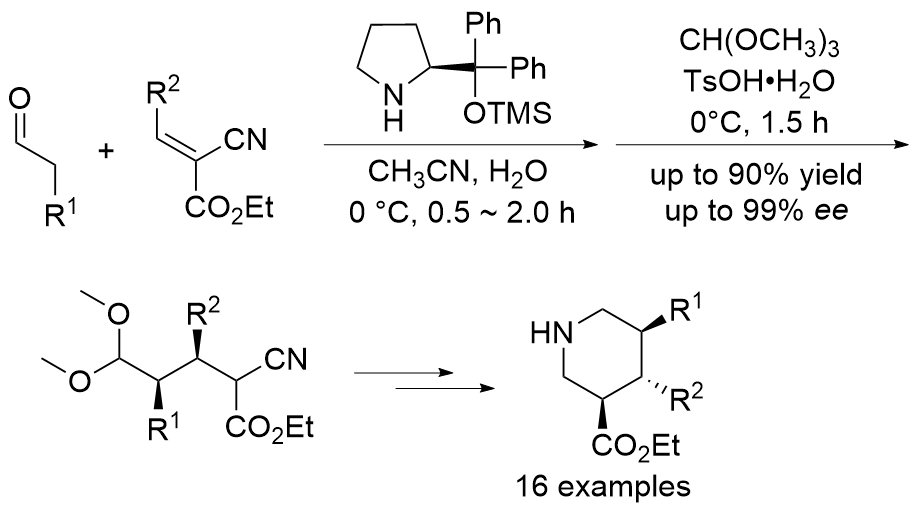


| [1] |
Chen, M. Y.; Liang, Y. C.; Dong, T. T.; Liang, W. J.; Liu, Y. P.; Zhang, Y. G.; Huang, X.; Kong, L. C.; Wang, Z.-X.; Peng, B. Angew. Chem. Int. Ed. 2021, 60, 2339.
|
| [2] |
Ji, J.-M.; Zhou, H. R.; Eom, Y. K.; Kim, C. H.; Kim, H. K. Adv. Energy Mater. 2020, 10, 2000124.
|
| [3] |
Zeng, Q.; Chen, Y. M.; Yan, Y. Y.; Wan, R.; Li, Y. J.; Fu, H. L.; Liu, Y.; Liu, S.; Yan, X.-X.; Cui, M. C. Anal. Chem. 2022, 94, 15261.
|
| [4] |
Zagata, A.; Traskovskis, K.; Belyakov, S.; Mihailovs, I.; Bundulis, A.; Rutkis, M. Dyes Pigm. 2022, 208, 110864.
|
| [5] |
Ye, H.; Ke, Y. J.; Li, W. L.; Zhu, B. T.; Jiang, L. R.; Hu, X. C.; Zeng, L. T. Anal. Chim. Acta 2023, 1254, 341125.
|
| [6] |
Kleine-Kleffmann, L.; Schulz, A.; Stepanenko, V.; Würthner, F. Angew. Chem. Int. Ed. 2024, 62, e202314667.
|
| [7] |
Wang, J. Q; Wang, C. Y; Wang, Y. F; Qiao, J. W; Ren, J. Z; Li, J. Y; Wang, W. X.; Chen, Z. H; Yu, Y.; Hao, X. T; Zhang, S. Q; Hou, J. H. Angew. Chem. Int. Ed. 2024, 63, e20240056.
|
| [8] |
Duan, Z. Q.; Liu, M.; Zheng, B.; Tang, Y.; Du, J.; Wang, L.; Yu, S. C.; Wu, Y. J.; Guo, H. C. Org. Lett. 2023, 25, 3298.
|
| [9] |
Hu, C.-X.; Chen, L.; Hu, D.; Song, X.; Chen, Z.-C.; Du, W.; Chen, Y.-C. Org. Lett. 2022, 22, 8973.
|
| [10] |
Gao, C.-F.; Zhou, Y.; Ma, H.; Zhang, Y.; Nie, J.; Zhang, F.-G.; Ma, J.-A. CCS Chem. 2022, 4, 3693.
|
| [11] |
Gaviña, D.; Escolano, M.; Sotorríos, L.; Gómez-Bengoa, E.; López- Ortiz, F.; Sánchez-Roselló, M.; Pozo, C. D. Adv. Synth. Catal. 2023, 365, 1.
|
| [12] |
Chen, H.; Sun, S. H.; Liu, Y. A.; Liao, X. B. ACS Catal. 2020, 10, 1397.
|
| [13] |
Park, J.; Kim, T.-I.; Cho, S.; Pandith, A.; Kim, Y. Biomaterials 2022, 289, 121749.
|
| [14] |
Wang, K.; Lin, X. F.; Li, Q.; Liu, Y.; Li, C. Chin. J. Catal. 2022, 43, 1812.
doi: 10.1016/S1872-2067(21)64051-2 |
| [15] |
Curti, C.; Battistini, L.; Sartori, A.; Zanardi, F. Chem. Rev. 2020, 120, 2448.
|
| [16] |
Cui, H. L.; Chen, Y. C. Chem. Commun. 2009, 30, 4479.
|
| [17] |
Mu, Y. C.; Nguyen, T. T.; Koh, M. J.; Schrock, R. R.; Hoveyda, A. H. Nat. Chem. 2019, 11, 478.
|
| [18] |
Shcherban, N. D.; Mäki-Arvela, P.; Aho, A.; Sergiienko, S. A.; Yaremov, P. S.; Eränen, K.; Murzin, D.Y. Catal. Sci. Technol. 2018, 8, 2928.
|
| [19] |
Gui, Q.-W.; Teng, F.; Li, Z.-C.; Jin, X.-F.; Zhang, M.; Dai, J.-N.; Lin, Y. W.; Cao, Z.; He, W. M. Org. Chem. Front. 2020, 7, 4026.
|
| [20] |
Miao, Z. C.; Luan, Y.; Qi, C.; Ramella, D. Dalton Trans. 2016, 45, 13917.
|
| [21] |
Meng, D.; Qiao, Y. S.; Wang, X.; Wen, W.; Zhao, S. H. RSC Adv. 2018, 8, 30180.
|
| [22] |
Nguyen, B. N.; Tran, M. H.; Bui, T. T. T.; Mac, D. H.; Pham, V.; Retailleau, P.; Nguyen, T. B. J. Org. Chem. 2023, 88, 11197.
|
| [23] |
Mydlova, L.; Taboukhat, S.; Waszkowska, K.; Ibrahim, N.; Migalska- Zalas, A.; Sahraoui, B.; Frère, P.; Makowska-Janusik, M. J. Mol. Liq. 2020, 314, 113622.
|
| [24] |
Yang, L.; Chen, S. Y.; Yi, D.; Chen, Q. X.; Zhang, J.; Xie, Y. S.; Sun, H. Y. J. Mater. Chem. B 2021, 9, 8512.
doi: 10.1039/d1tb01189h pmid: 34554170 |
| [25] |
Anbu, N.; Maheswari, R.; Elamathi, V.; Varalakshmi, P.; Dhakshinamoorthy, A. Catal. Commun. 2020, 138, 105954.
|
| [26] |
Wilk, M.; Trzepizur, D.; Koszelewski, D.; Brodzka, A.; Ostasze- wski, R. Bioorg. Chem. 2019, 93, 102816.
|
| [27] |
Lolak, N.; Kuyuldar, E.; Burhan, H.; Goksu, H.; Akocak, S.; Sen, F. ACS Omega 2019, 4, 6848.
|
| [28] |
Yang, Y.; Wang, D.; Jiang, P. F.; Gao, W. L.; Cong, R. H.; Yang, T. Mol. Catal. 2020, 490, 110914.
|
| [29] |
Wang, W. F.; Luo, M.; Yao, W. W.; Ma, M. T.; Pullarkat, S. A.; Xu, L.; Leung, P.-H. Chem. Eng. 2019, 7, 1718.
|
| [30] |
Takakura, R.; Koyama, K.; Kuwata, M.; Yamada, T.; Sajiki, H.; Sawama, Y. Org. Biomol. Chem. 2020, 18, 6594.
doi: 10.1039/d0ob01397h pmid: 32813006 |
| [31] |
Watson, G.; Derakhshandeh, P. G.; Abednatanzi, S.; Schmidt, J.; Leus, K.; Van der Voort, P. Molecules, 2021, 26, 838.
|
| [32] |
Abedian-Dehaghani, N.; Sadjadi, S.; Heravi, M. M. Sci. Rep. 2022, 12, 16395.
doi: 10.1038/s41598-022-20699-2 pmid: 36180555 |
| [33] |
Sakata, N.; Sasakura, K.; Matsushita, G.; Okamoto, K.; Ohe, K. Org. Lett. 2017, 19, 3422.
doi: 10.1021/acs.orglett.7b01378 pmid: 28657324 |
| [34] |
Zhang, Y.; Wei, Y.; Li, S.; Ma, J.-A. Eur. J. Org. Chem. 2019, 2019, 199.
doi: 10.1002/ejoc.201801504 |
| [35] |
Li, Z.; Liu, C.; Wang, J.; Wang, S. J.; Xiao, L. W.; Jing, X. M. Spectrochim. Acta, Part A 2019, 212, 349.
|
| [36] |
Sarveswari, S.; Beneto, A. J.; Siva, A. Sens. Actuators, B 2017, 245, 428.
|
| [37] |
Cesaretti, A.; Foggi, P.; Fortuna, C. G.; Elisei, F.; Spalletti, A.; Carlotti, B. J. Phys. Chem. C 2020, 124, 15739.
|
| [38] |
Khalid, M.; Khan, M. U.; Shafiq, I.; Hussain, R.; Ali, A.; Imran, M.; Braga, A. A. C.; Rehman, M. F, U.; Akram, M. S. R. Soc. Open Sci. 2021, 8, 210570.
|
| [39] |
Lv, G. L.; Xu, Y. Z.; Yang, J. J.; Li, W. H.; Li, C. X.; Sun, A. Y. Analyst 2020, 145, 6579.
|
| [40] |
Arunkumar, A.; Shanavas, S.; Acevedo, R.; Anbarasan, P. M. Opt. Quantum Electron. 2020, 52, 164.
|
| [41] |
Gong, P. P.; An, L. L.; Tong, J. F.; Liu, X. P.; Liang, Z. Z.; Li, J. F. Nanomaterials 2022, 12, 3700.
|
| [42] |
Yahya, M.; Seferoglu, N.; Barsella, A.; Achelle, S.; Seferoglu, Z. Spectrochim. Acta, Part A 2021, 248, 119178.
|
| [43] |
Yu, H.; Fang, Y.; Wang, J.; Zhang, Q.; Chen, S. J.; Wang, K.-P.; Hu, Z.-Q. Anal. Bioanal. Chem. 2022, 414, 6779.
|
| [44] |
Wang, Z.-Y.; Cao, X.-Y.; Li, Z.-H.; Shen, Q. CN 117664934, 2023.
|
| [45] |
Battal, A.; Kassa, S. B.; Gultekin, N. A.; Tavasli, M.; Onganer, Y. Spectrochim. Acta, Part A 2023, 304, 123350.
|
| [46] |
Huang, J. J.; Zhou, Y. H.; Wang, W. X.; Zhu, J. M.; Li, X. C.; Fang, M.; Wu, Z. Y.; Zhu, W. J.; Li, C. Spectrochim. Acta, Part A 2023, 286, 122011.
|
| [47] |
Ramesh, S.; Kumaresan, S. Microchem. J. 2021, 169, 106584.
|
| [48] |
Popczyk, A.; Chereta, Y.; El-Ghayourya, A.; Sahraoui, B.; Mysliwiec, J. Dyes Pigm. 2020, 177, 108300.
|
| [49] |
Chen, Z.-H.; Yu, S.-W.; Xu, W.-J.; Li, M.-X.; Zeng, Y.; Deng, S.-W.; Lin, J.-Y.; Wang, Z.-Y. Organics, 2024, 5, 46.
|
| [50] |
Tu, L. P.; Liu, J.; Zhang, Z. C.; Qi, Q. R.; Yao, S.; Huang, W. C. Analyst 2021, 146, 2221.
|
| [51] |
Jia, Y.; Guan, W.-L.; Liu, J.; Hu, J.-P.; Shi, B. B.; Yao, H.; Zhang, Y.-M.; Wei, T.-B.; Lin, Q. Chin. Chem. Lett. 2023, 34, 108082.
|
| [52] |
Shan, X. F.; Wu, S. T.; Shen, L. Y.; Peng, S. L.; Xu, H.; Wang, Z.-Y.; Yang, X.-J.; Huang, Y.-L.; Feng, X.; Redshaw, C.; Zhang, Q.-L. Dyes Pigm. 2023, 219, 111609.
|
| [53] |
Wang, B.-W.; Jiang, K.; Li, J.-X.; Luo, S.-H.; Wang, Z.-Y.; Jiang, H.-F. Angew. Chem. Int. Ed. 2020, 59, 2338.
|
| [54] |
Chen, S.-H.; Chen, Z.-H.; Jiang, K.; Cao, X.-Y.; Chen, L.-Y.; Ouyang, J.; Wang, Z.-Y. Spectrochim. Acta, Part A 2023, 300, 122905.
|
| [55] |
Xue, J.; Tang, F.; Ding, A. X.; He, F.; Huang, J. Y.; Kong, L.; Yang, J. X. J. Lumin. 2022, 250, 119119.
|
| [56] |
Delgado-Montiel, T.; Baldenebro-López, J.; Soto-Rojo, R.; Glossman- Mitnik, D. Molecules 2020, 25, 3670.
|
| [57] |
Özarslan, A.; Çakmaz, D.; Erol, F.; Senöz, H.; Seferoglu, N.; Barsella, A.; Seferoglu, Z. J. Mol. Struct. 2021, 1229, 129583.
|
| [58] |
Mustafa, F. M.; Abdel-Latif, M. K.; Abdel-Khalek, A. A.; Kühn, O. Molecules, 2023, 28, 5185.
|
| [59] |
Han, L.; Chen, Q. Q.; Yu, H. D.; Lu, Y.; Jiang, S. L. J. Photochem. Photobiol. A 2021, 416, 113341.
|
| [60] |
Duvva, N.; Eom, Y. K.; Reddy, G.; Schanze, K. S.; Giribabu, L. ACS Appl. Energy Mater. 2020, 3, 6758.
|
| [61] |
Al-Marhabi, A. R.; El-Shishtawy, R. M.; Bouzzine, S. M.; Hamidi, M.; Al-Ghamdi, H. A.; Al-Footy, K. O. J. Photochem. Photobiol. A 2023, 436, 114389.
|
| [62] |
Xu, D. Q.; Tong, H. S.; Shen, J.; Qiu, W. W.; Qian, L. S. Chin. J. Org. Chem. 2024, 44, 1240 (in Chinese).
|
|
(徐冬青, 童海姗, 沈杰, 邱万伟, 钱立生, 有机化学, 2024, 44, 1240.)
doi: 10.6023/cjoc202309008 |
|
| [63] |
Kula, S.; Szlapa-Kula, A.; Fabianczyk, A.; Gnida, P.; Libera, M.; Bujak, K.; Siwy, M.; Schab-Balcerzak, E. J. Photochem. Photobiol. B 2019, 197, 111555.
|
| [64] |
Xu, C.-J.; Li, H.-W.; He, X.-L.; Du, W.; Chen, Y.-C. Asian J. Org. Chem. 2019, 8, 1037.
|
| [65] |
Manneveau, M.; Tanii, S.; Gens, F.; Legros, J.; Chataigner, I. Org. Biomol. Chem. 2020, 18, 3481.
doi: 10.1039/d0ob00582g pmid: 32347286 |
| [66] |
Yuan, J. W.; Shen, L.; Guo, N.; Yin, Y. L.; Yang, P. Y.; Yang, L. R.; Xiao, Y. M.; Zhang, S. R. J. Org. Chem. 2023, 88, 16598.
|
| [67] |
Wang, N.; Yan, X.; Hu, Z.-T.; Feng, Y.; Zhu, L.; Chen, Z.-H.; Wang, H.; Wang, Q.-L.; Ouyang, Q.; Zheng, P.-F. Org. Lett. 2022, 24, 8553.
doi: 10.1021/acs.orglett.2c03578 pmid: 36377976 |
| [68] |
Ben Hassen, M.; Masmoudi, F.; Zribi, L.; Trigui, M.; Ismaili, L.; Marco-Contelles, J.; Chabchoub, F. ChemistrySelect 2021, 6, 945.
doi: 10.1002/slct.202004577 |
| [69] |
Hayashi, Y.; Kranidiotis-Hisatomi, N.; Sakamoto, D.; Oritani, K.; Kawamoto, T.; Kamimura, A. Eur. J. Org. Chem. 2019, 2018, 6843.
|
| [70] |
Hayashi, Y.; Odoh, A. S.; Kranidiotis-Hisatomi, N. ChemCatChem 2020, 12, 2412.
|
| [71] |
Li, F.; Wang, X.; Liu, Y. M.; Zhang, J.; Yang, J. Synth. Commun. 2020, 50, 56.
|
| [72] |
Zhang, W.; Tang, C.-S.; Xiang, S.-Q. RSC Adv. 2022, 12, 29840.
doi: 10.1039/d2ra04936h pmid: 36321094 |
| [73] |
Lu, C. J.; Ye, M.; Long, L. P.; Zheng, Y.; Liu, J. M.; Zhang, Y.; Chen, Z. W. J. Org. Chem. 2022, 87, 1545.
|
| [74] |
Hu, L. J.; Lin, S. X.; Li, S. W.; Kang, Q.; Du, Y. ChemCatChem 2020, 12, 118.
|
| [75] |
Chen, X. J.; Zhao, Y. J.; Huang, C.; Zhao, Z. F.; Zhao, W. W.; Li, S.-W. Chem. Commun. 2024, 60, 236.
|
| [76] |
Bojanowski, J.; Albrecht, A. ChemistrySelect 2019, 4, 4588.
doi: 10.1002/slct.201900579 |
| [77] |
Rout, S.; Ray, S. K.; Unhale, R. A.; Singh, V. K. Org. Lett. 2014, 16, 5568.
|
| [78] |
Martelli, L. S. R.; Vieira, L. C. C.; Paixao, M. W.; Zukerman- Schpector, J.; de Souza, J. O.; Aguiar, A. C. C.; Oliva, G.; Guido, R. V. C.; Corrêa, A. G. Tetrahedron 2019, 75, 3530.
doi: 10.1016/j.tet.2019.05.022 |
| [79] |
Hayashi, Y.; Han, X. L.; Mori, N. Chem. Eur. J. 2023, 29, e202301093.
|
| [80] |
Wu, H.-C.; Wang, C.; Chen, Y.-H.; Liu, Y.-K. Chem. Commun. 2021, 57, 1762.
|
| [81] |
Wang, Y.; Niu, C.; Xie, D.-H.; Du, D.-M. Org. Biomol. Chem. 2021, 19, 8572.
doi: 10.1039/d1ob01256h pmid: 34549755 |
| [82] |
Ittamalla, C.; Chintha, S.; Medishetti, N.; Nanubolu, J. B.; Atmakur, K. Eur. J. Org. Chem. 2023, 26, e202300357.
|
| [83] |
Cao, L.; Li, J.-X.; Wu, H.-Q.; Jiang, K.; Hao, Z.-F.; Luo, S.-H.; Wang, Z.-Y. ACS Sustainable Chem. Eng. 2018. 6, 4147.
|
| [84] |
Uchikura, T.; Toda, M.; Mouri, T.; Fujii, T.; Moriyama, K.; Ibáñez, I.; Akiyama, T. J. Org. Chem. 2020, 85, 12715.
|
| [85] |
Pálvölgyi, A. M.; Ehrschwendtner, F.; Schnürch, M.; Bica-Schröder, K. Org. Biomol. Chem. 2022, 20, 7245.
doi: 10.1039/d2ob01364a pmid: 36073152 |
| [86] |
Yang, M.-L.; Dong, C.-L.; Guan, Z.; He, Y.-H. J. Org. Chem. 2024, 89, 1285.
|
| [87] |
Li, L.; Guo, S.; Wang, Q.; Zhu, J. Org. Lett. 2019, 21, 5462.
|
| [88] |
Matsumoto, A.; Yamamoto, M.; Maruoka, K. ACS Catal. 2022, 12, 2045.
|
| [89] |
Jia, Q. F.; Lan, Y. F.; Ye, X.; Lin, Y. H.; Ren, Q. RSC Adv. 2020, 10, 29171.
|
| [90] |
Liu, M.; Zhou, L. J.; Shi, W. Y.; Hu, Y. M.; Liao, J. N.; Duan, Z. Q.; Wang, W.; Wu, Y. J.; Zheng, B.; Guo, H. C. Org. Lett. 2021, 23, 7703.
|
| [91] |
Gao, Y.; Song, X.; Yan, R.-J.; Du, W.; Chen, Y.-C. Org. Biomol. Chem. 2021, 19, 151.
|
| [92] |
Zhang, J.; Xiong, D. K.; Jiang, Z. W.; Chen, S. T.; Huang, G.-B.; Li, J. S.; Wang, Z. M.; Yang, J. G. Org. Lett. 2024, 26, 1447.
doi: 10.1021/acs.orglett.4c00078 pmid: 38353475 |
| [93] |
Yang, D. Z.; Zhu, M.; Wang, T. M.; He, Y. X.; Xie, L.; Zhang, J. Y.; Cheng, B. Org. Biomol. Chem. 2023, 21, 5413.
|
| [94] |
Yuan, C.; Tan, H.; Jiang, X.-F.; Liu, S.; Jiang, L.; Cao, Q.-Y.; Xu, X.-J.; Deng, X.-Y.; Pan, G.-N.; Chen, J.-Y.; Cui, H.-L. Asian J. Org. Chem. 2019, 8, 1893.
|
| [95] |
Dai, C. L.; Li, M. S.; Chen, M. J.; Luo, N. L.; Wang, C. D. J. Chem. Res. 2019, 43, 43.
|
| [96] |
Kowalska, E.; Dyguda, M.; Artelska, A.; Albrecht, A. J. Org. Chem. 2023, 88, 16589.
doi: 10.1021/acs.joc.3c02172 pmid: 38037694 |
| [97] |
Radwan, M. F.; Elboray, E. E.; Dardeer, H. M.; Kobayashi, Y.; Furuta, T.; Hamada, S.; Dohi, T.; Aly, M. F. Chem. Asian J. 2023, 18, e202300215.
|
| [98] |
Zhou, Y.; Gao, C.-F.; Ma, H.; Nie, J.; Ma, J.-A.; Zhang, F.-G.; Zhang, Y. CCS Chem. 2022, 4, 3693.
|
| [99] |
Lukasik, B.; Romaniszyn, M.; Kloszewski, N.; Albrecht, L. Org. Lett. 2023, 25, 3728.
|
| [100] |
Romaniszyn, M.; Gronowska, K.; Albrecht, L. J. Org. Chem. 2019, 84, 9929.
doi: 10.1021/acs.joc.9b01091 pmid: 31244164 |
| [101] |
Romaniszyn, M.; Sieron, L.; Albrecht, L. J. Org. Chem. 2022, 87, 5296.
|
| [102] |
Obydennov, D. L.; Simbirtseva, A. E.; Piksin, S. E.; Sosnovskikh, V. Y. ACS Omega 2020, 5, 33406.
doi: 10.1021/acsomega.0c05357 pmid: 33403303 |
| [103] |
Xie, X. F.; Wang, L.; Zhou, Q.; Ma, Y. M.; Wang, Z.-M.; Li, P. H. Chin. Chem. Lett. 2022, 33, 5069.
|
| [104] |
Guo, M. Z.; Dong, F. Y.; Yin, X. C.; Xu, L. B.; Wang, L.; Li, S.-S. Org. Chem. Front. 2021, 8, 2224.
|
| [105] |
Wang, N.; Lin, J.-Y.; Luo, S.-H.; Zhou, Y.-J.; Yang, K.; Chen, R.-H.; Yang, G.-X.; Wang, Z.-Y. Amino Acids 2022, 54, 989.
|
| [106] |
Zhou, Y.-J.; Fang, Y.-G.; Yang, K.; Yu, S.-W.; Chen, Z.-J.; Wang, B.-C.; Zhan, H.-Y.; Wang, Z.-Y. Asian J. Org. Chem. 2023, 12, e202300038.
|
| [107] |
Zhou, Y.-J.; Fang, Y.-G.; Yang, K.; Lin, J.-Y.; Li, H.-Q.; Chen, Z.-J.; Wang, Z.-Y. Molecules 2023, 28, 5635.
|
| [108] |
Kornfeind, J.; Allen, James E.; Keller, Taylor M.; Fleming, Fraser F. J. Org. Chem. 2023, 88, 15947.
doi: 10.1021/acs.joc.3c02210 pmid: 37938807 |
| [109] |
Liu, Q.-P.; Chen, X. Y.; Yu, N.; Li, Y.-L.; He, K.-C.; Zheng, W.-H.; Zhou, Y.-Q.; Jiang, K.; Yang, L. M.; Wei, Y. ACS Catal. 2023, 13, 5795.
|
| [110] |
Suzuki, I.; Kasahara, N.; Ogura, K.; Shibata, I. Chem.-Eur. J. 2024, 30, e202302365.
|
| [111] |
Suzuki, I.; Ogura, K.; Shimazu, J.; Shibata, I. Eur. J. Org. Chem. 2021, 2021, 2785.
|
| [112] |
Suzuki, I.; Tsunoi, S.; Shibata, I. Org. Lett. 2017, 19, 2690.
doi: 10.1021/acs.orglett.7b01023 pmid: 28459596 |
| [113] |
Suzuki, I.; Shimazu, J. -Y.; Tsunoi, S.; Shibata, I. Eur. J. Org. Chem. 2019, 2019, 3658.
doi: 10.1002/ejoc.201900518 |
| [114] |
Zhang, Y. J.; Shi, S. K.; Yang, Z. H. J. Org. Chem. 2024, 89, 2748.
|
| [115] |
Yang, Z. M.; Z, T. T.; Cao, B. Y.; Liao, D. J.; Hu, X. Y.; Zhao, S. L.; Qin, J. K. Mater. Today Chem. 2024, 35, 101890.
|
| [116] |
Xiao, F. F.; Lei, D.; Liu, C. G.; Li, Y. S.; Ren, W. F.; Li, J. G.; Li, D. Z.; Zu, B. Y.; Dou, X. C. Angew. Chem. Int. Ed. 2024, 63, e20240045.
|
| [117] |
Mwalukuku, V. M.; Liotier, J.; Riquelme, A. J.; Kervella, Y.; Huaul- me, Q.; Haurez, A.; Narbey, S.; Anta, J. A.; Demadrille, R. Adv. Energy Mater. 2023, 13, 2203651.
|
| [118] |
Chen, Z. H.; Zhang, S. Q.; Zhang, T.; Ren, J. Z.; Dai, J. B.; Li, H. X.; Qiao, J. W.; Hao, X. T.; Hou, J. H. Angew. Chem. Int. Ed. 2024, 63, e202317892.
|
| [1] | 何春, 刘姝祺, 成奋民, 郝远强, 陈述, 张培盛, 曾荣今. 具有双态发光性质的双光子丹磺酰类荧光探针的合成及其在硫化氢检测中的应用研究[J]. 有机化学, 2024, 44(9): 2869-2875. |
| [2] | 苏小龙, 李健鹏, 刘孟鑫, 邹莉, 杨得锁, 冯海涛. 四苯乙烯酰胺类化合物的合成及其高灵敏度、高选择性识别Cu2+[J]. 有机化学, 2024, 44(8): 2581-2587. |
| [3] | 胡亦然, 张巳亮, 罗海艳, 赵璐瑶, 郭旭东, 王双青, 胡睿, 杨国强. 新型查尔酮衍生物氨肽酶N荧光探针的设计及应用[J]. 有机化学, 2024, 44(7): 2257-2264. |
| [4] | 刘琳, 陈琳, 胡晓玲, 钟克利, 张璟琳, 汤立军. 基于苯并吡喃点亮型硫化氢荧光探针在食品检测中的应用与细胞成像[J]. 有机化学, 2024, 44(6): 2027-2032. |
| [5] | 张文艳, 王丹, 罗仁洁, 刘会玲. 近红外荧光手术导航探针的研究进展[J]. 有机化学, 2024, 44(6): 1760-1776. |
| [6] | 李非凡, 余康, 倪传志, 朱园园, 曾婕, 古双喜. 测定氨基酸浓度和对映体组成的手性荧光探针[J]. 有机化学, 2024, 44(6): 1862-1869. |
| [7] | 张继东, 杨垚, 张杰, 厍伟. 基于聚集诱导效应(AIE)-激发态分子内质子转移(ESIPT)效应的四苯乙烯荧光探针对Zn(II)检测研究[J]. 有机化学, 2024, 44(4): 1337-1342. |
| [8] | 程晓红, 刘发龙, 孙进博, 张锐. 一种用于次氯酸根实时高灵敏度检测的络合物基荧光探针[J]. 有机化学, 2024, 44(4): 1284-1292. |
| [9] | 徐冬青, 童海姗, 沈杰, 邱万伟, 钱立生. 脂滴特异性荧光探针的构建及可视化肝肿瘤细胞[J]. 有机化学, 2024, 44(4): 1240-1246. |
| [10] | 张莹珍, 江丹丹, 李娟华, 王菁菁, 刘昆明, 刘晋彪. 高选择性硒代半胱氨酸荧光探针的构建策略及成像[J]. 有机化学, 2024, 44(1): 41-53. |
| [11] | 杨维清, 葛宴兵, 陈元元, 刘萍, 付海燕, 马梦林. 1,8-萘酰亚胺衍生物的设计、合成及其对半胱氨酸的识别研究[J]. 有机化学, 2024, 44(1): 180-194. |
| [12] | 李焕清, 陈兆华, 陈祖佳, 邱琪雯, 张又才, 陈思鸿, 汪朝阳. 基于有机小分子的汞离子荧光探针研究进展[J]. 有机化学, 2023, 43(9): 3067-3077. |
| [13] | 丁炳辉, 韩少辉, 熊海青, 王本花, 左伯军, 宋相志. 高选择性比率型荧光探针用于急性肺损伤中次氯酸的检测[J]. 有机化学, 2023, 43(8): 2878-2884. |
| [14] | 李宜芳, 王耀, 牛华伟, 陈秀金, 李兆周, 王永国. 线粒体靶向的二氧化硫荧光探针研究进展[J]. 有机化学, 2023, 43(6): 1952-1962. |
| [15] | 刘甜甜, 张鸿鹏, 焦晓梦, 白银娟. 多信号同时检测生物硫醇荧光探针的研究进展[J]. 有机化学, 2023, 43(6): 2081-2095. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||