Chinese Journal of Organic Chemistry ›› 2019, Vol. 39 ›› Issue (10): 2965-2972.DOI: 10.6023/cjoc201903031 Previous Articles Next Articles
NOTES
张学博, 马航宇, 孙腾达, 雷鹏, 杨新玲, 张晓鸣, 凌云*( )
)
收稿日期:2019-03-17
修回日期:2019-04-18
发布日期:2019-05-21
通讯作者:
凌云
E-mail:lyun@cau.edu.cn
基金资助:
Zhang, Xuebo, Ma, Hangyu, Sun, Tengda, Lei, Peng, Yang, Xinling, Zhang, Xiaoming, Ling, Yun*( )
)
Received:2019-03-17
Revised:2019-04-18
Published:2019-05-21
Contact:
Ling, Yun
E-mail:lyun@cau.edu.cn
Supported by:Share
Zhang, Xuebo, Ma, Hangyu, Sun, Tengda, Lei, Peng, Yang, Xinling, Zhang, Xiaoming, Ling, Yun. Design, Synthesis and Fungicidal Activity of Novel Piperidine Containing Cinnamaldehyde Thiosemicarbazide Derivatives[J]. Chinese Journal of Organic Chemistry, 2019, 39(10): 2965-2972.
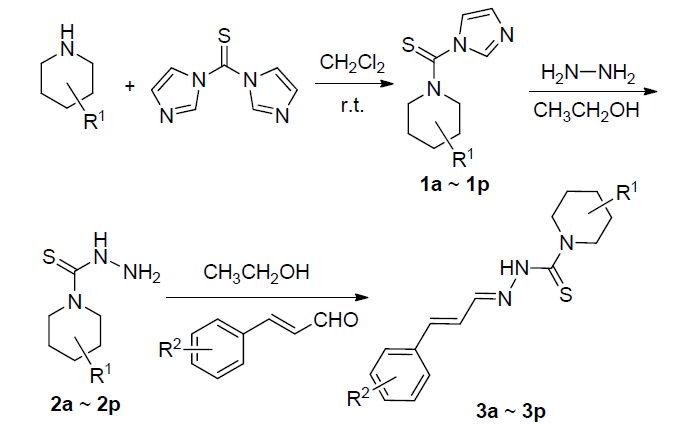
| Compd. | R1 | R2 | 瓜果腐霉病 | 水稻纹枯病 | 苹果腐烂病 | 油菜菌核病 | 水稻恶苗病 |
|---|---|---|---|---|---|---|---|
| 3a | H | 4-Cl | 96 | 100 | 99 | 99 | 68 |
| 3b | H | 2-CH3 | 46 | 78 | 91 | 47 | 44 |
| 3c | H | 2-OCH3 | 93 | 100 | 100 | 70 | 63 |
| 3d | H | 4-OCH3 | 94 | 84 | 95 | 99 | 100 |
| 3e | H | 4-NO2 | 80 | 85 | 95 | 66 | 53 |
| 3f | 4-COOC2H5 | 4-Cl | 92 | 100 | 78 | 59 | 49 |
| 3g | 4-COOC2H5 | 2-CH3 | 30 | 71 | 55 | 54 | 35 |
| 3h | 4-COOC2H5 | 2-OCH3 | 95 | 100 | 91 | 47 | 51 |
| 3i | 4-COOC2H5 | 4-OCH3 | 86 | 82 | 85 | 69 | 79 |
| 3j | 4-COOC2H5 | 4-NO2 | 82 | 88 | 80 | 51 | 73 |
| 3k | 3-CH3 | 4-Cl | 83 | 100 | 98 | 89 | 60 |
| 3l | 3-CH3 | 2-CH3 | 31 | 76 | 80 | 42 | 45 |
| 3m | 3-CH3 | 2-OCH3 | 85 | 100 | 98 | 81 | 54 |
| 3n | 3-CH3 | 4-OCH3 | 84 | 81 | 95 | 98 | 94 |
| 3o | 3-CH3 | 4-NO2 | 70 | 81 | 93 | 77 | 75 |
| 3p | H | H | 100 | 95 | 98 | 100 | 95 |
| A | 44 | 100 | 91 | 62 | 49 | ||
| P | 35 | 87 | 71 | 49 | 45 | ||
| Lead compound | 99 | 93 | 86 | 85 | 64 | ||
| 吡唑醚菌酯 | 47 | 100 | 100 | 100 | 79 | ||
| Compd. | R1 | R2 | 瓜果腐霉病 | 水稻纹枯病 | 苹果腐烂病 | 油菜菌核病 | 水稻恶苗病 |
|---|---|---|---|---|---|---|---|
| 3a | H | 4-Cl | 96 | 100 | 99 | 99 | 68 |
| 3b | H | 2-CH3 | 46 | 78 | 91 | 47 | 44 |
| 3c | H | 2-OCH3 | 93 | 100 | 100 | 70 | 63 |
| 3d | H | 4-OCH3 | 94 | 84 | 95 | 99 | 100 |
| 3e | H | 4-NO2 | 80 | 85 | 95 | 66 | 53 |
| 3f | 4-COOC2H5 | 4-Cl | 92 | 100 | 78 | 59 | 49 |
| 3g | 4-COOC2H5 | 2-CH3 | 30 | 71 | 55 | 54 | 35 |
| 3h | 4-COOC2H5 | 2-OCH3 | 95 | 100 | 91 | 47 | 51 |
| 3i | 4-COOC2H5 | 4-OCH3 | 86 | 82 | 85 | 69 | 79 |
| 3j | 4-COOC2H5 | 4-NO2 | 82 | 88 | 80 | 51 | 73 |
| 3k | 3-CH3 | 4-Cl | 83 | 100 | 98 | 89 | 60 |
| 3l | 3-CH3 | 2-CH3 | 31 | 76 | 80 | 42 | 45 |
| 3m | 3-CH3 | 2-OCH3 | 85 | 100 | 98 | 81 | 54 |
| 3n | 3-CH3 | 4-OCH3 | 84 | 81 | 95 | 98 | 94 |
| 3o | 3-CH3 | 4-NO2 | 70 | 81 | 93 | 77 | 75 |
| 3p | H | H | 100 | 95 | 98 | 100 | 95 |
| A | 44 | 100 | 91 | 62 | 49 | ||
| P | 35 | 87 | 71 | 49 | 45 | ||
| Lead compound | 99 | 93 | 86 | 85 | 64 | ||
| 吡唑醚菌酯 | 47 | 100 | 100 | 100 | 79 | ||
| Compd. | EC50/(μg?mL–1) | 线性方程 | R2 |
|---|---|---|---|
| 3a | 5.53 | y=2.2927x+3.2971 | 0.98 |
| 3b | 15.17 | y=2.2541x+2.3389 | 0.97 |
| 3c | 8.56 | y=1.7871x+3.3347 | 0.98 |
| 3d | 6.09 | y=1.6526x+3.7034 | 0.98 |
| 3e | 17.60 | y=0.9497x+3.8172 | 0.95 |
| 3f | 16.62 | y=1.3833x+3.3123 | 0.99 |
| 3h | 14.23 | y=1.3574x+3.4357 | 0.97 |
| 3i | 51.81 | y=0.8067x+3.6170 | 0.97 |
| 3j | 24.72 | y=1.2658x+3.2367 | 0.91 |
| 3k | 5.22 | y=1.8442x+3.6777 | 0.98 |
| 3l | 8.64 | y=1.8229x+3.2947 | 0.99 |
| 3m | 6.04 | y=1.4877x+3.8395 | 0.98 |
| 3n | 33.53 | y=0.9639x+3.5296 | 0.98 |
| 3o | 19.95 | y=0.9814x+3.7242 | 0.93 |
| 3p | 5.20 | y=2.0812x+3.5097 | 0.96 |
| Lead compound | 6.58 | y=1.8537x+3.4834 | 0.98 |
| A | 5.70 | y=1.3559x+3.9746 | 0.99 |
| P | 16.70 | y=1.0628x+3.7005 | 0.96 |
| 嘧菌酯 | 0.01 | y=0.7677x+6.4285 | 0.94 |
| 吡唑醚菌酯 | 0.01 | y=1.2443x+7.4561 | 0.96 |
| Compd. | EC50/(μg?mL–1) | 线性方程 | R2 |
|---|---|---|---|
| 3a | 5.53 | y=2.2927x+3.2971 | 0.98 |
| 3b | 15.17 | y=2.2541x+2.3389 | 0.97 |
| 3c | 8.56 | y=1.7871x+3.3347 | 0.98 |
| 3d | 6.09 | y=1.6526x+3.7034 | 0.98 |
| 3e | 17.60 | y=0.9497x+3.8172 | 0.95 |
| 3f | 16.62 | y=1.3833x+3.3123 | 0.99 |
| 3h | 14.23 | y=1.3574x+3.4357 | 0.97 |
| 3i | 51.81 | y=0.8067x+3.6170 | 0.97 |
| 3j | 24.72 | y=1.2658x+3.2367 | 0.91 |
| 3k | 5.22 | y=1.8442x+3.6777 | 0.98 |
| 3l | 8.64 | y=1.8229x+3.2947 | 0.99 |
| 3m | 6.04 | y=1.4877x+3.8395 | 0.98 |
| 3n | 33.53 | y=0.9639x+3.5296 | 0.98 |
| 3o | 19.95 | y=0.9814x+3.7242 | 0.93 |
| 3p | 5.20 | y=2.0812x+3.5097 | 0.96 |
| Lead compound | 6.58 | y=1.8537x+3.4834 | 0.98 |
| A | 5.70 | y=1.3559x+3.9746 | 0.99 |
| P | 16.70 | y=1.0628x+3.7005 | 0.96 |
| 嘧菌酯 | 0.01 | y=0.7677x+6.4285 | 0.94 |
| 吡唑醚菌酯 | 0.01 | y=1.2443x+7.4561 | 0.96 |
| Compd. | EC50/(μg?mL–1) | 线性方程 | R2 |
|---|---|---|---|
| 3a | 2.35 | y=1.3663x+4.4942 | 0.96 |
| 3c | 2.20 | y=0.9405x+4.6784 | 0.95 |
| 3d | 6.17 | y=1.1077x+4.1249 | 0.97 |
| 3e | 6.80 | y=1.1765x+4.0206 | 0.99 |
| 3f | 4.04 | y=1.4348x+4.1311 | 0.97 |
| 3h | 3.66 | y=1.2256x+4.3109 | 0.98 |
| 3i | 7.77 | y=1.2411x+3.8950 | 0.95 |
| 3j | 8.60 | y=1.5194x+3.5798 | 0.99 |
| 3k | 1.22 | y=0.9178x+4.9219 | 0.95 |
| 3m | 1.46 | y=1.1335x+4.8152 | 0.99 |
| 3n | 10.49 | y=1.1615x+3.8146 | 0.94 |
| 3o | 10.22 | y=1.2466x+3.7418 | 0.95 |
| 3p | 1.02 | y=0.9761x+4.9881 | 0.98 |
| Lead compound | 5.60 | y=0.6868x+4.4864 | 0.91 |
| A | 3.12 | y=1.3133x+4.3346 | 0.92 |
| P | 5.60 | y=0.9895x+4.2629 | 0.91 |
| 吡唑醚菌酯 | 0.03 | y=0.7435x+6.1470 | 0.99 |
| Compd. | EC50/(μg?mL–1) | 线性方程 | R2 |
|---|---|---|---|
| 3a | 2.35 | y=1.3663x+4.4942 | 0.96 |
| 3c | 2.20 | y=0.9405x+4.6784 | 0.95 |
| 3d | 6.17 | y=1.1077x+4.1249 | 0.97 |
| 3e | 6.80 | y=1.1765x+4.0206 | 0.99 |
| 3f | 4.04 | y=1.4348x+4.1311 | 0.97 |
| 3h | 3.66 | y=1.2256x+4.3109 | 0.98 |
| 3i | 7.77 | y=1.2411x+3.8950 | 0.95 |
| 3j | 8.60 | y=1.5194x+3.5798 | 0.99 |
| 3k | 1.22 | y=0.9178x+4.9219 | 0.95 |
| 3m | 1.46 | y=1.1335x+4.8152 | 0.99 |
| 3n | 10.49 | y=1.1615x+3.8146 | 0.94 |
| 3o | 10.22 | y=1.2466x+3.7418 | 0.95 |
| 3p | 1.02 | y=0.9761x+4.9881 | 0.98 |
| Lead compound | 5.60 | y=0.6868x+4.4864 | 0.91 |
| A | 3.12 | y=1.3133x+4.3346 | 0.92 |
| P | 5.60 | y=0.9895x+4.2629 | 0.91 |
| 吡唑醚菌酯 | 0.03 | y=0.7435x+6.1470 | 0.99 |
| Compd. | EC50/(μg?mL–1) | 线性方程 | R2 |
|---|---|---|---|
| 3a | 4.94 | y=0.8204x+4.4318 | 0.91 |
| 3d | 7.41 | y=2.1292x+3.1484 | 0.91 |
| 3k | 7.55 | y=1.0553x+4.0749 | 0.99 |
| 3m | 9.57 | y=1.3686x+3.6580 | 0.94 |
| 3n | 47.43 | y=1.1461x+3.0791 | 0.91 |
| 3p | 5.10 | y=2.8944x+2.9508 | 0.94 |
| Lead compound | 12.61 | y=0.7568x+4.1669 | 0.94 |
| A | 24.21 | y=1.1892x+3.3541 | 0.98 |
| P | 60.40 | y=1.0606x+3.1111 | 0.92 |
| 嘧菌酯 | 5.17 | y=0.5026x+4.6425 | 0.91 |
| Compd. | EC50/(μg?mL–1) | 线性方程 | R2 |
|---|---|---|---|
| 3a | 4.94 | y=0.8204x+4.4318 | 0.91 |
| 3d | 7.41 | y=2.1292x+3.1484 | 0.91 |
| 3k | 7.55 | y=1.0553x+4.0749 | 0.99 |
| 3m | 9.57 | y=1.3686x+3.6580 | 0.94 |
| 3n | 47.43 | y=1.1461x+3.0791 | 0.91 |
| 3p | 5.10 | y=2.8944x+2.9508 | 0.94 |
| Lead compound | 12.61 | y=0.7568x+4.1669 | 0.94 |
| A | 24.21 | y=1.1892x+3.3541 | 0.98 |
| P | 60.40 | y=1.0606x+3.1111 | 0.92 |
| 嘧菌酯 | 5.17 | y=0.5026x+4.6425 | 0.91 |
| Compd. | EC50/(μg?mL–1) | 线性方程 | R2 |
|---|---|---|---|
| 3a | 9.04 | y=2.1430x+2.9500 | 0.93 |
| 3c | 35.15 | y=1.3196x+2.9617 | 0.98 |
| 3d | 4.35 | y=1.2912x+4.1757 | 0.94 |
| 3e | 23.72 | y=2.1148x+2.0921 | 0.97 |
| 3f | 26.43 | y=2.9907x+0.7485 | 0.92 |
| 3h | 22.21 | y=3.0688x+0.8690 | 0.95 |
| 3i | 10.12 | y=1.0758x+3.9186 | 0.91 |
| 3j | 18.27 | y=1.5940x+2.9888 | 0.97 |
| 3k | 21.65 | y=2.6595x+1.4494 | 0.98 |
| 3m | 17.72 | y=2.2467x+2.1962 | 0.94 |
| 3n | 10.49 | y=1.5598x+3.1988 | 0.99 |
| 3p | 8.40 | y=4.9150x+0.4688 | 0.92 |
| Lead compound | 5.37 | y=2.3678x+3.2722 | 0.90 |
| A | 50.00 | y=1.0501x+3.2140 | 0.92 |
| P | 189.60 | y=0.6994x+3.4069 | 0.93 |
| 嘧菌酯 | 16.88 | y=0.3656x+4.5528 | 0.92 |
| Compd. | EC50/(μg?mL–1) | 线性方程 | R2 |
|---|---|---|---|
| 3a | 9.04 | y=2.1430x+2.9500 | 0.93 |
| 3c | 35.15 | y=1.3196x+2.9617 | 0.98 |
| 3d | 4.35 | y=1.2912x+4.1757 | 0.94 |
| 3e | 23.72 | y=2.1148x+2.0921 | 0.97 |
| 3f | 26.43 | y=2.9907x+0.7485 | 0.92 |
| 3h | 22.21 | y=3.0688x+0.8690 | 0.95 |
| 3i | 10.12 | y=1.0758x+3.9186 | 0.91 |
| 3j | 18.27 | y=1.5940x+2.9888 | 0.97 |
| 3k | 21.65 | y=2.6595x+1.4494 | 0.98 |
| 3m | 17.72 | y=2.2467x+2.1962 | 0.94 |
| 3n | 10.49 | y=1.5598x+3.1988 | 0.99 |
| 3p | 8.40 | y=4.9150x+0.4688 | 0.92 |
| Lead compound | 5.37 | y=2.3678x+3.2722 | 0.90 |
| A | 50.00 | y=1.0501x+3.2140 | 0.92 |
| P | 189.60 | y=0.6994x+3.4069 | 0.93 |
| 嘧菌酯 | 16.88 | y=0.3656x+4.5528 | 0.92 |
| [1] |
Godfray, H. C. J.; Beddington, J. R.; Crute, I. R.; Haddad, L.; Lawrence, D.; Muir, J. F.; Pretty, J.; Robinson, S.; Thomas, S. M.; Toulmin, C . Science 2010, 327, 812.
doi: 10.1126/science.1185383 |
| [2] |
Gerwick, B. C.; Sparks, T. C. Pest Manage. Sci. 2014, 70, 1169.
doi: 10.1002/ps.2014.70.issue-8 |
| [3] |
Balba, H. J. Environ. Sci. Health, Part B 2007, 42, 441.
doi: 10.1080/03601230701316465 |
| [4] |
Sparks, T. C.; Hahn, D. R.; Garizi, N. V. Pest Manage. Sci. 2017, 73, 700.
doi: 10.1002/ps.2017.73.issue-4 |
| [5] | Ashakirin, S. N.; Tripathy, M.; Patil, U. K.; Abdul Majeed, A. B. Int. J. Pharm. Sci. Res. 2017, 8, 2333. |
| [6] |
Ka, H.; Park, H. J.; Jung, H. J.; Choi, J. W.; Cho, K. S.; Ha, J.; Lee, K. T. Cancer Lett. 2003, 196, 143.
doi: 10.1016/S0304-3835(03)00238-6 |
| [7] |
Chang, S. T.; Chen, P. F.; Chang, S. C. J. Ethnopharmacol. 2001, 77, 123.
doi: 10.1016/S0378-8741(01)00273-2 |
| [8] |
Zhu, Y. J.; Song, K. K.; Li, Z. C.; Pan, Z. Z.; Guo, Y. J.; Zhou, J. J.; Wang, Q.; Liu, B.; Chen, Q. X. J. Agric. Food Chem. 2009, 57, 5518.
doi: 10.1021/jf9007554 |
| [9] |
Gutiérrez, L.; Escudero, A.; Batlle, R.; Nerín, C. J. Agric. Food Chem. 2009, 57, 8564.
doi: 10.1021/jf901459e |
| [10] |
Du, W. X.; Avena-Bustillos, R. J.; Woods, R.; Breksa, A. P.; McHugh, T. H.; Friedman, M.; Levin, C. E.; Mandrell, R. J. Agric. Food Chem. 2012, 60, 7799.
doi: 10.1021/jf301281a |
| [11] | Niknahad, H.; Shuhendler, A.; Galati, G.; Siraki, A. G.; Easson, E.; Poon, R.; O'Brien, P. J. Chem. Biol. Interact. 2003, 143, 119. |
| [12] |
Cheng, S. S.; Liu, J. Y.; Tsai, K. H.; Chen, W. J.; Chang, S. T. J. Agric. Food Chem. 2004, 52, 4395.
doi: 10.1021/jf0497152 |
| [13] |
Yu, Y.; Kalinowski, D. S.; Kovacevic, Z.; Siafakas, A. R.; Jansson, P. J.; Stefani, C.; Lovejoy, D. B.; Sharpe, P. C.; Bernhardt, P. V.; Richardson, D. R. J. Med. Chem. 2009, 52, 5271.
doi: 10.1021/jm900552r |
| [14] |
Dong, H.; Liu, J.; Liu, X.; Yu, Y.; Cao, S. J. Mol. Struct. 2018, 1151, 353.
doi: 10.1016/j.molstruc.2017.08.034 |
| [15] |
Dong, H.; Liu, J.; Liu, X.; Yu, Y.; Cao, S . Bioorg. Chem. 2017, 75, 106.
doi: 10.1016/j.bioorg.2017.07.002 |
| [16] |
Liu, L. F.; Li, P. Y.; Qian, Q. Q.; Lei, X. L.; Huang, Y. X. Chin. J. Org. Chem. 2013, 33, 854 (in Chinese).
doi: 10.6023/cjoc201301027 |
|
( 刘利锋, 李培源, 钱全全, 雷晓琳, 黄钰湘, 周泉, 黄珊, 肖琦, 苏炜, 有机化学, 2013, 33, 854.)
doi: 10.6023/cjoc201301027 |
|
| [17] |
Xu, Y.; Wang, Z.; Dong, W.; Xing, J.; Liang, P.; Yang, X. L. Chin. J. Org. Chem. 2012, 32, 1278 (in Chinese).
doi: 10.6023/cjoc1111181 |
|
( 徐焱, 王振, 凌云, 董玮, 邢静, 梁沛, 杨新玲, 有机化学, 2012, 32, 1278.)
doi: 10.6023/cjoc1111181 |
|
| [18] |
Zhang, X. B.; Lei, P.; Sun, T. D; Jin, X. Y.; Yang, X. L.; Ling, Y . Molecules 2017, 22, 2085.
doi: 10.3390/molecules22122085 |
| [19] | Zhang, X. M.; Lei, P.; Li, X. L.; Yang, X. L.; Zhang, X. B.; Sun, T. D.; Ling, Y. Chin. J. Org. Chem. 2019, 39, 3197 (in Chinese). |
| ( 张晓鸣, 雷鹏, 李欣潞, 杨新玲, 张学博, 孙腾达, 凌云, 有机化学, 2019, 39, 3197.) |
| [1] | Fakai Zou, Nengzhong Wang, Hui Yao, Hui Wang, Mingguo Liu, Nianyu Huang. Regio- and Stereo-selective Synthesis of 1β-/3R-Aryl Thiosugar [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 593-604. |
| [2] | Luyao Li, Zhongwen He, Zhenguo Zhang, Zhenhua Jia, Teck-Peng Loh. Application of Triaryl Carbenium in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 421-437. |
| [3] | Qinggang Mei, Qinghan Li. Recent Progress of Visible Light-Induced the Synthesis of C(3) (Hetero)arylthio Indole Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 398-408. |
| [4] | Penghui Li, Qingyang Xie, Fuxian Wan, Yuanhong Zhang, Lin Jiang. Synthesis and Fungicidal Activity of Novel Substituted Pyrimidine-5-carboxamides Bearing Cyclopropyl Moiety [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 650-656. |
| [5] | Weiqing Yang, Yanbing Ge, Yuanyuan Chen, Ping Liu, Haiyan Fu, Menglin Ma. Design and Synthesis of Fluorescent 1,8-Napthalimide Derivatives and Their Identification of Cysteine [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 180-194. |
| [6] | Qianfan Zhao, Yongzheng Chen, Shiming Zhang. Application and Mechanism Study of Carbon-Based Metal-Free Catalysts in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 137-147. |
| [7] | Shan Chen, Zhilin Chen, Qiong Hu, Yanshuang Meng, Yue Huang, Pingfang Tao, Liru Lu, Guobao Huang. Recognition of Bis-thiourea Tweezers to Neutral Molecules in Non-Polar Solvent [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 277-281. |
| [8] | Huakun Wang, Xiaolong Ren, Yining Xuan. Study of the Halide Salt Catalyzed [3+2] Cycloaddition of α,β-Epoxy Carboxylate with Isocyanate [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 251-258. |
| [9] | Yukun Jin, Baoyi Ren, Fushun Liang. Visible Light-Mediated Selective C—F Bond Cleavage of Trifluoromethyl Groups and Its Application in Synthesizing gem-Difluoro-Containing Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 85-110. |
| [10] | Cuiyun Ma, Hailan Luo, Fuhua Zhang, Dan Guo, Shuxing Chen, Fei Wang. Green Biosynthesis, Photophysical Properties and Application of 3-Pyrrolyl BODIPY [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 216-223. |
| [11] | Bozhen Wang, Jie Zhang, Chunhui Nian, Mingming Jin, Miaomiao Kong, Wulan Li, Wenfei He, Jianzhang Wu. Synthesis and Antitumor Activity of 3,4-Dichlorophenyl Amides [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 232-241. |
| [12] | Shihang Yu, Jiawei Liu, Biyu An, Qinghua Bian, Min Wang, Jiangchun Zhong. Asymmetric Synthesis of the Contact Sex Pheromone of Neoclytus acuminatus acuminatus (Fabricius) [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 301-308. |
| [13] | Weizhong Ding, Bingwen Zhang, Yanqing Xue, Yuqi Lin, Zhijun Tang, Jing Wang, Wenchao Yang, Xiaofeng Wang, Wen Liu. A New Polyketide from Fusarium graminearum [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3319-3322. |
| [14] | Yang Li, Jinding Yuan, Di Zhao. Deep Eutectic Solvent of 1,3-Dimethylurea/L-(+)-Tartaric Acid for the Green Synthesis of (E)-2-Styrylquinoline-3-carboxylic Acid Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3268-3276. |
| [15] | Dandan Sui, Nannan Cen, Ruoqu Gong, Yang Chen, Wenbo Chen. Supporting-Electrolyte-Free Electrochemical Synthesis of Trifluoromethylated Oxindoles in Continuous Flow [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3239-3245. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||