Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (3): 1081-1097.DOI: 10.6023/cjoc202008024 Previous Articles Next Articles
Special Issue: 热点论文虚拟合集
REVIEWS
收稿日期:2020-08-13
修回日期:2020-09-03
发布日期:2020-09-22
通讯作者:
吴磊
基金资助:
Jie Zhua, Wenchao Yangb, Chengyun Zhanga, Lei Wua,*( )
)
Received:2020-08-13
Revised:2020-09-03
Published:2020-09-22
Contact:
Lei Wu
About author:Supported by:Share
Jie Zhu, Wenchao Yang, Chengyun Zhang, Lei Wu. Recent Progress in the Synthesis of Dendralenes: A Decade Update[J]. Chinese Journal of Organic Chemistry, 2021, 41(3): 1081-1097.

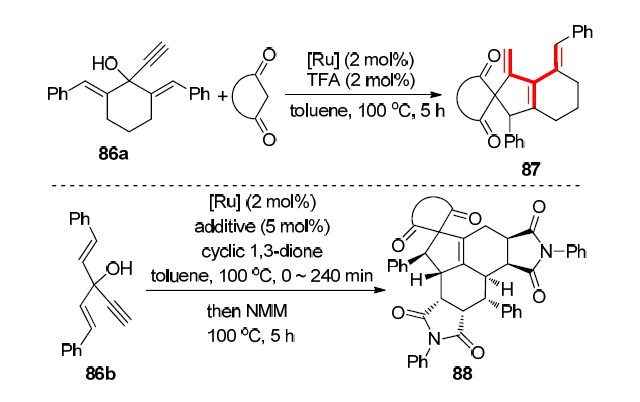
| [1] |
(a) Hopf, H. Angew. Chem.. Int. Ed. 1984, 23, 948.
pmid: 26151489 |
|
(b) Hopf, H. Nature 2009, 460, 183.
doi: 10.1038/460183a pmid: 26151489 |
|
|
(c) Hopf, H. Angew. Chem., Int. Ed. 2001, 40, 705.
doi: 10.1002/1521-3773(20010216)40:4【-逻*辑*与-】amp;lt;【-逻*辑*与-】amp;gt;1.0.CO;2-X pmid: 26151489 |
|
|
(d) Sherburn, M. S. Acc. Chem. Res. 2015, 48, 1961.
doi: 10.1021/acs.accounts.5b00242 pmid: 26151489 |
|
|
(e) Hopf, H.; Sherburn, M. S. Cross Conjugation-Modern Dendralene, Radialene and Fulvene Chemistry, Wiley-VCH, Verlag GmbH & Co. KGaA, 2016.
pmid: 26151489 |
|
| [2] |
Hopf, H. Classics in Hydrocarbon Chemistry: Syntheses, Concepts, Perspectives, Wiley-VCH, Weinheim, 2000, p. 103.
|
| [3] |
Paul, R.; Tchelitcheff, S. C. R. Hebd. Seances Acad. Sci. 1951, 232, 1939.
|
| [4] |
(a) Bolmquist, A. T.; Verdol, J. A. J. Am. Chem. Soc. 1955, 77, 81;.
doi: 10.1021/ja01606a025 |
|
(b) Bailey, W. J.; Ecomomy, J. J. Am. Chem. Soc. 1955, 77, 1133;.
doi: 10.1021/ja01610a014 |
|
|
(c) Bailey, W. J.; Nielsen, N. A. J. Org. Chem. 1962, 27, 3088.
doi: 10.1021/jo01056a025 |
|
| [5] |
(a) Bradford, T. A.; Payne, A. D.; Willis, A. C.; Paddon-Row, M. N.; Sherburn, M. S. J. Org. Chem. 2010, 75, 491.
doi: 10.1021/jo9024557 pmid: 20000615 |
|
(b) Hopf, H.; Sherburn, M. S. Angew. Chem.. Int. Ed. 2012, 51, 2298.
doi: 10.1002/anie.v51.10 pmid: 20000615 |
|
|
(c) Naidua, G. S.; Singh, R. Ghosh, S. K. Synlett 2018, 29, 282.
doi: 10.1055/s-0036-1590960 pmid: 20000615 |
|
| [6] |
Fielder, S.; Rowan, D. D.; Sherburn, M. S. Angew. Chem., Int. Ed. 2000, 39, 4331.
doi: 10.1002/(ISSN)1521-3773 |
| [7] |
Brummond, K. M.; Chen, H.; Sill, P.; You, L. J. Am. Chem. Soc. 2002, 124, 15186.
doi: 10.1021/ja027588p pmid: 12487589 |
| [8] |
Miller, N. A.; Willis, A. C.; Paddon-Row, M. N.; Sherburn, M. S. Angew. Chem., Int. Ed. 2007, 46, 937.
doi: 10.1002/(ISSN)1521-3773 |
| [9] |
Payne, A. D.; Bojase, G.; Paddon-Row, M. N.; Sherburn, M. S. Angew. Chem., Int. Ed. 2009, 48, 4836.
doi: 10.1002/anie.v48:26 |
| [10] |
(a) Stehling, L.; Wilke, G. Angew. Chem.. Int. Ed. 1988, 27, 571.
doi: 10.1002/(ISSN)1521-3773 pmid: 32559086 |
|
(b) Payne, A. D.; Willis, A. C.; Sherburn, M. S. J. Am. Chem. Soc. 2005, 127, 12188.
doi: 10.1021/ja053772+ pmid: 32559086 |
|
|
(c) Pellissier, H. Tetrahedron 2005, 61, 6479.
doi: 10.1016/j.tet.2005.04.014 pmid: 32559086 |
|
|
(d) Frontier, A. J.; Collison, C. Tetrahedron 2005, 61, 7577.
doi: 10.1016/j.tet.2005.05.019 pmid: 32559086 |
|
|
(e) Tius, M. A. Eur. J. Org. Chem. 2005,2193.
pmid: 32559086 |
|
|
(f) Pronin, S. V.; Shenvi, R. A. J. Am. Chem. Soc. 2012, 134, 19604;. 39ef1d2f-797f-4223-b3ac-b34dd9f23b4f
doi: 10.1021/0310129b pmid: 32559086 |
|
|
(g) Fallon, T.; Willis, A. C.; Paddon-Row, M. N.; Sherburn, M. S. J. Org. Chem. 2014, 79, 3185.
doi: 10.1021/jo500458y pmid: 32559086 |
|
|
(h) Desfeux, C.; Besnard, C.; Mazet, C. Org. Lett. 2020, 22, 8181.
doi: 10.1021/acs.orglett.0c01892 pmid: 32559086 |
|
| [11] |
(a) Bloomquist, A. T.; Verdol, J. A. J. Am. Chem. Soc. 1955, 77, 81.
doi: 10.1021/ja01606a025 pmid: 11671638 |
|
(b) Bailey, W. J.; Economy, J. J. Am. Chem. Soc. 1955, 77, 1133.
doi: 10.1021/ja01610a014 pmid: 11671638 |
|
|
(c) Bloomquist, A. T.; Verdol, J. A. J. Am. Chem. Soc. 1955, 77, 1806.
doi: 10.1021/ja01612a026 pmid: 11671638 |
|
|
(d) Bailey, W. J.; Cunov, C. H.; Nicholas, L. J. Am. Chem. Soc. 1955, 77, 2787.
doi: 10.1021/ja01615a034 pmid: 11671638 |
|
|
(e) Martin, H. D.; Echert-Macsic, M.; Mayer, B. Angew. Chem.. Int. Ed. 1980, 19, 807.
pmid: 11671638 |
|
|
(f) Hopf, H. Angew. Chem.. Int. Ed. 1982, 21, 286.
pmid: 11671638 |
|
|
(g) Hopf, H. Angew. Chem.. Int. Ed. 1984, 96, 947.
doi: 10.1002/(ISSN)1521-3757 pmid: 11671638 |
|
|
(h) Brain, P. T.; Smart, B. A.; Robertson, H. E.; Davis, M. J.; H. Rankin, D. W.; Henry, W. J.; Gosney, I. J. Org. Chem. 1997, 62, 2767.
doi: 10.1021/jo962091h pmid: 11671638 |
|
|
(i) Woo, S.; Squires, N.; Fallis, A. G. Org. Lett. 1999, 1, 573.
doi: 10.1021/ol990695c pmid: 11671638 |
|
|
(j) Woo, S.; Legoupy, S.; Parra, S.; Fallis, A. G. Org. Lett. 1999, 1, 1013.
doi: 10.1021/ol990798v pmid: 11671638 |
|
|
(k) Le Nôtre, J.; Martinez,, A. A.; Dixneuf,, P. H.; Bruneau,, C. Tetrahedron 2003, 59, 9425.
doi: 10.1016/j.tet.2003.09.073 pmid: 11671638 |
|
|
(l) Park, S.; Lee, D. Synthesis 2007,2313.
pmid: 11671638 |
|
| [12] |
(a) Shimizu, M.; Kurahashi, T.; Shimono, K.; Tanaka, K.; Nagao, I.; Kiyomoto, S.; Hiyama, T. Chem.-Asian J. 2007, 2, 1400.
doi: 10.1002/asia.200700120 pmid: 17828718 |
|
(b) Bojase, G.; Payne, A. D.; Willis, A. C.; Sherburn, M. S. Angew. Chem.. Int. Ed. 2008, 47, 910.
doi: 10.1002/(ISSN)1521-3773 pmid: 17828718 |
|
| [13] |
George, J.; Ward, J. S.; Sherburn, M. S. Chem. Sci. 2019, 10, 9969.
doi: 10.1039/c9sc03976g pmid: 32055353 |
| [14] |
Ghosh, S. K.; Singh, R.; Date, S. M. Chem. Commun. 2003,636.
|
| [15] |
Singh, R.; Ghosh, S. K. Chem. Commun. 2011, 47, 10809.
doi: 10.1039/c1cc14211a |
| [16] |
(a) Singh, R.; Naidu, G. S.; Ghosh, S. K. Proc. Natl. Acad. Sci.. India, Sect. A Phys. Sci. 2016, 86, 619.
doi: 10.1007/s40010-016-0300-2 |
|
(b) Naidu, G. S.; Singh, R.; Kumarb, M.; Ghosh, S. K. RSC Adv. 2016, 6, 37136.
doi: 10.1039/C6RA01729K |
|
| [17] |
Rahif, M.; Roux, M.; Thibonnet, J.; Parrain, J.-L. Mol. Diversity 2013, 17, 49.
doi: 10.1007/s11030-012-9418-6 |
| [18] |
(a) Ma, S. Chem. Rev. 2005, 105, 2829.
doi: 10.1021/cr020024j pmid: 24479609 |
|
(b) Ye, J.; Ma, S. Acc. Chem. Res. 2014, 47, 989.
doi: 10.1021/ar4002069 pmid: 24479609 |
|
|
(c) Jia, M.; Ma, S. Angew. Chem.. Int. Ed. 2016, 55, 9134.
doi: 10.1002/anie.v55.32 pmid: 24479609 |
|
| [19] |
Wang, H.; Beiring, B.; Yu, D.-G.; Collins, K. D.; Glorius, F. Angew. Chem., Int. Ed. 2013, 52, 12430.
doi: 10.1002/anie.201306754 |
| [20] |
Qiu, Y.; Posevins, D.; Bäckvall, J.-E. Angew. Chem., Int. Ed. 2017, 56, 13112.
doi: 10.1002/anie.201706211 |
| [21] |
Zhang, T.; Song, C.; Meng, Y.; Chen, P.; Xu, H.; Chang, J. J. Org. Chem. 2017, 82, 9905.
doi: 10.1021/acs.joc.7b01710 pmid: 28816455 |
| [22] |
(a) Lu, X.-Y.; Zhang, C.-M.; Xu, Z.-R. Acc. Chem. Res. 2001, 34, 535.
doi: 10.1021/ar000253x pmid: 19847345 |
|
(b) Cowen, B. J.; Miller, C. J. Chem. Soc. Rev. 2009, 38, 3102.
doi: 10.1039/b816700c pmid: 19847345 |
|
|
(c) Pei, C.-K. Shi, M. Chem.-Eur. J. 2012, 18, 6712. 42d445b1-4d74-44a8-ae66-c143c537e548
doi: 10.1002/chem.201200209 pmid: 19847345 |
|
| [23] |
(a) Yang, Y.; Qiu, X.; Zhao, Y.; Mu, Y.; Shi, Z. J. Am. Chem. Soc. 2016, 138, 495.
doi: 10.1021/jacs.5b11569 pmid: 26709532 |
|
(b) Yang, Y.; Li, R.; Zhao, Y.; Zhao, D.; Shi, Z. J. Am. Chem. Soc. 2016, 138, 8743.
pmid: 26709532 |
|
| [24] |
Mao, M.; Zhang, L.; Chen, Y.-Z.; Zhu, J.; Wu, L. ACS Catal. 2017, 7, 181.
doi: 10.1021/acscatal.6b02972 |
| [25] |
Xia, Y.-T.; Xie, X.-Y.; Cui, S.-H.; Ji, Y.-G.; Wu, L. Chem. Commun. 2019, 55, 11699.
doi: 10.1039/C9CC05928H |
| [26] |
Xia, Y.-T.; Wu, J.-J.; Zhang, C.-Y.; Mao, M.; Ji, Y.-G.; Wu, L. Org. Lett. 2019, 21, 6383.
doi: 10.1021/acs.orglett.9b02287 pmid: 31356086 |
| [27] |
Rivera-Chao, E.; Fañanás-Mastral, M. Angew. Chem., Int. Ed. 2018, 57, 9945.
doi: 10.1002/anie.v57.31 |
| [28] |
Li, H.; Gontla, R.; Flegel, J.; Merten, C.; Ziegler, S.; Antonchick, A. P.; Waldmann, H. Angew. Chem., Int. Ed. 2019, 58, 307.
doi: 10.1002/anie.201811041 |
| [29] |
Frank, B. B.; Kivala, M.; Blanco, B. C.; Breiten, B.; Schweizer, W. B.; Laporta, P. R.; Biaggio, I.; Jahnke, E.; Tykwinski, R. R.; Boudon, C.; Gisselbrecht, J.-P.; Diederich, F. Eur. J. Org. Chem. 2010,2487.
|
| [30] |
Yamauchi, T.; Shibata, Y.; Aki, T.; Yoshimura, A.; Yao, M.; Misaki, Y. Chem. Lett. 2018, 47, 1176.
doi: 10.1246/cl.180496 |
| [31] |
Januszewski, J. A.; Hampel, F.; Neiss, C.; Gçrling, A.; Tykwinski, R. R. Angew. Chem., Int. Ed. 2014, 53, 3743.
doi: 10.1002/anie.v53.14 |
| [32] |
Saglam, M. F.; Fallon, T.; Paddon-Row, M. N.; Sherburn, M. S. J. Am. Chem. Soc. 2016, 138, 1022.
doi: 10.1021/jacs.5b11889 pmid: 26721640 |
| [33] |
Polák, P.; Tobrman, T. Eur. J. Org. Chem. 2019,957.
|
| [34] |
Lippincott, D. J.; Linstadt, R. T. H.; Maser, M. R.; Lipshutz, B. H. Angew. Chem., Int. Ed. 2017, 56, 847.
doi: 10.1002/anie.v56.3 |
| [35] |
(a) Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457.
doi: 10.1021/cr00039a007 pmid: 20028025 |
|
(b) Kennedy, J. W. J.; Hall, D. G. J. Am. Chem. Soc. 2002, 124, 11586.
doi: 10.1021/ja027453j pmid: 20028025 |
|
|
(c) Ishiyama, T.; Miyaura, N. Chem. Rec. 2004, 3, 271.
doi: 10.1002/tcr.10068 pmid: 20028025 |
|
|
(d) Clay, J. M.; Vedejs, E. J. Am. Chem. Soc. 2005, 127, 5766.
doi: 10.1021/ja043743j pmid: 20028025 |
|
|
(e) Mkhalid, I. A. I.; Barnard, J. H.; Marder, T. B.; Murphy, J. M.; Hartwig, J. F. Chem. Rev. 2010, 110, 890.
doi: 10.1021/cr900206p pmid: 20028025 |
|
| [36] |
Deng, Y.; Bartholomeyzik, T.; Persson, A. K. Å.; Sun, J.; Bäckvall, J.-E. Angew. Chem., Int. Ed. 2012, 51, 2703.
doi: 10.1002/anie.201107592 |
| [37] |
Deng, Y.; Bartholomeyzik, T.; Bäckvall, J.-E. Angew. Chem., Int. Ed. 2013, 52, 6283.
doi: 10.1002/anie.201301167 |
| [38] |
Bartholomeyzik, T.; Pendrill, R.; Lihammar, R.; Jiang, T.; Widmalm, G.; Bäckvall, J.-E. J. Am. Chem. Soc. 2018, 140, 298.
doi: 10.1021/jacs.7b10267 pmid: 29155573 |
| [39] |
Volla, C.; M., R.; Bäckvall, J.-E. Angew. Chem., Int. Ed. 2013, 52, 14209.
doi: 10.1002/anie.201308448 |
| [40] |
Volla, C. M. R.; Mazuela, J.; Bäckvall, J.-E. Chem.-Eur. J. 2014, 20, 7608. 612af94e-9bb6-475a-b483-a43afb87ebb1
doi: 10.1002/chem.201402688 |
| [41] |
Yang, B.; Qiu, Y.; Bäckvall, J.-E. Acc. Chem. Res. 2018, 51, 1520.
doi: 10.1021/acs.accounts.8b00138 pmid: 29792667 |
| [42] |
Volla, C. M. R.; Bäckvall, J.-E. ACS Catal. 2016, 6, 6398.
doi: 10.1021/acscatal.6b02070 pmid: 27761298 |
| [43] |
Zhu, C.; Yang, B.; Qiu, Y.; Bäckvall, J.-E. Angew. Chem., Int. Ed. 2016, 55, 14405.
doi: 10.1002/anie.v55.46 |
| [44] |
Naidu, V. R.; Posevins, D.; Volla, C. M. R.; Bäckvall, J.-E. Angew. Chem., Int. Ed. 2017, 56, 1590.
doi: 10.1002/anie.v56.6 |
| [45] |
Jonek, A.; Berger, S.; Haak, E. Chem.-Eur. J. 2012, 18, 15504. 626a977e-2498-4edb-bb1b-ae34a1d2499a
doi: 10.1002/chem.201202414 |
| [46] |
Thies, N.; Haak, E. Angew. Chem., Int. Ed. 2015, 54, 4097.
doi: 10.1002/anie.201412207 |
| [47] |
Sakashita, K.; Shibata, Y.; Tanaka, K. Angew. Chem., Int. Ed. 2016, 55, 6753.
doi: 10.1002/anie.201602155 |
| [48] |
Li, L.; Luo, P.; Deng, Y.; Shao, Z. Angew. Chem., Int. Ed. 2019, 58, 4710.
doi: 10.1002/anie.v58.14 |
| [1] | Baochang Gao, Yu Shi, Yuan Tian, Zhiguo Zhang, Jingru Zhang, Yufeng Sun, Guoliang Mao, Lingyan Dai. Synthesis of 4-Methyl-2-oxo-6-arylamino-2H-pyran-3-carbonitrile Derivatives [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 644-649. |
| [2] | Wenwen Chen, Qin Zhang, Songyue Zhang, Fangfang Huang, Xinyin Zhang, Jianfeng Jia. Visible Light Promoted Coupling Reaction of Alkynyl Iodide and Sodium Sulphinate without Photocatalyst [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 584-592. |
| [3] | Hong'en Tong, Hongyu Guo, Rong Zhou. Progress on Visible-Light Promoted Addition Reactions of Inert C—H Bonds to Carbonyls [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 54-69. |
| [4] | Yixin Jiang, Boxiao Tang, Haibo Mao, Xuexia Chen, Yangjie Yu, Cuiying Quan, Zhaoyang Xu, Jinhui Shi, Yilin Liu. A Green, Recyclable and Carrier-Free Study for the Coupling Reaction of Alkenes with Aryl Iodides in H2O-Polyethylene Glycol (PEG-200) [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3210-3215. |
| [5] | Jing Tang, Wenkun Luo, Jun Zhou. Advances in the Synthesis of Azaspiro[4.5]trienones [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3006-3034. |
| [6] | Zujia Chen, Shiwei Yu, Yongjun Zhou, Huanqing Li, Qiwen Qiu, Miaoxin Li, Zhaoyang Wang. Application of BF3•OEt2 in Organic Synthesis as a Catalyst or Synthon [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3107-3118. |
| [7] | Chunming Gui, Tongyao Zhou, Haifeng Wang, Qiongjiao Yan, Wei Wang, Jin Huang, Fener Chen. Recent Advances in Visible Light Photoredox-Catalyzed Alkynylation [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2647-2663. |
| [8] | Yingke Feng, He Wang, Mengxing Cui, Ran Sun, Xin Wang, Yang Chen, Lei Li. Visible-Light-Induced Difluoroalkylated Cyclization of Novel Functionalized Aromatic Isocyanides [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2913-2925. |
| [9] | Yuzhuo Chen, Hongmei Sun, Liang Wang, Fangzhi Hu, Shuaishuai Li. Research Progress on Construction of Heterocyclic Skeletons Based on α-Hydride Transfer Strategy [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2323-2337. |
| [10] | Rongbin Cai, Bing Li, Qi Zhou, Longyi Zhu, Jun Luo. Synthesis of 4,8,9,10-Tetrafunctionalized 2-Azaadamantanes and Their 2-Azaprotoadamantane Skeleton Isomers [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2217-2225. |
| [11] | Lixing Sun, Tingting Sun, Haiqing Wang, Shufang Wu, Xiaoye Wang, Tianya Liu, Yuchen Zhang. Lewis Acid-Catalyzed [2+4] Cyclization of 3-Alkyl-2-vinylindoles with α,β-Unsaturated N-Sulfonyl Ketimines [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2178-2188. |
| [12] | Zhijun Ren, Weiwei Luo, Jun Zhou. Recent Progress in Silver-Mediated Tandem Cyclization of N-Arylacrylamides [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2026-2039. |
| [13] | Yuxing Tong, Ziwei Wang, Ben Liu, Yaowei Xu, Song Gao, Xiangbing Tang, Xinghua Zhang. Recent Advances in Synthesis of 3-Sulfenylated Indoles [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1310-1324. |
| [14] | Shiquan Gao, Chuangjun Liu, Junfeng Yang, Junliang Zhang. Cobalt-Catalyzed Electrochemical Reductive Coupling of Alkynes and Alkenes [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1559-1565. |
| [15] | Jingpeng Li, Shuntao Huang, Qi Yang, Weiqiang Li, Teng Liu, Chao Huang. A Practical Synthesis of (Z)-N-Vinyl Substituted N,O-Acetals under Continuous Flow Technology [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1550-1558. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||