Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (1): 293-301.DOI: 10.6023/cjoc202105008 Previous Articles Next Articles
ARTICLES
尹丽君, 李超群, 吴晓霞, 徐广森, 李志颖, 沈月毛*( )
)
收稿日期:2021-05-06
修回日期:2021-06-06
发布日期:2021-06-29
通讯作者:
沈月毛
基金资助:
Lijun Yin, Chaoqun Li, Xiaoxia Wu, Guangsen Xu, Zhiying Li, Yuemao Shen( )
)
Received:2021-05-06
Revised:2021-06-06
Published:2021-06-29
Contact:
Yuemao Shen
Supported by:Share
Lijun Yin, Chaoqun Li, Xiaoxia Wu, Guangsen Xu, Zhiying Li, Yuemao Shen. Synthesis of (E)-N-(4-Styrene) Acrylamides for DNA Topoisomerase IIα Inhibitors and Antitumor Agents[J]. Chinese Journal of Organic Chemistry, 2022, 42(1): 293-301.
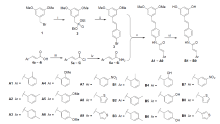
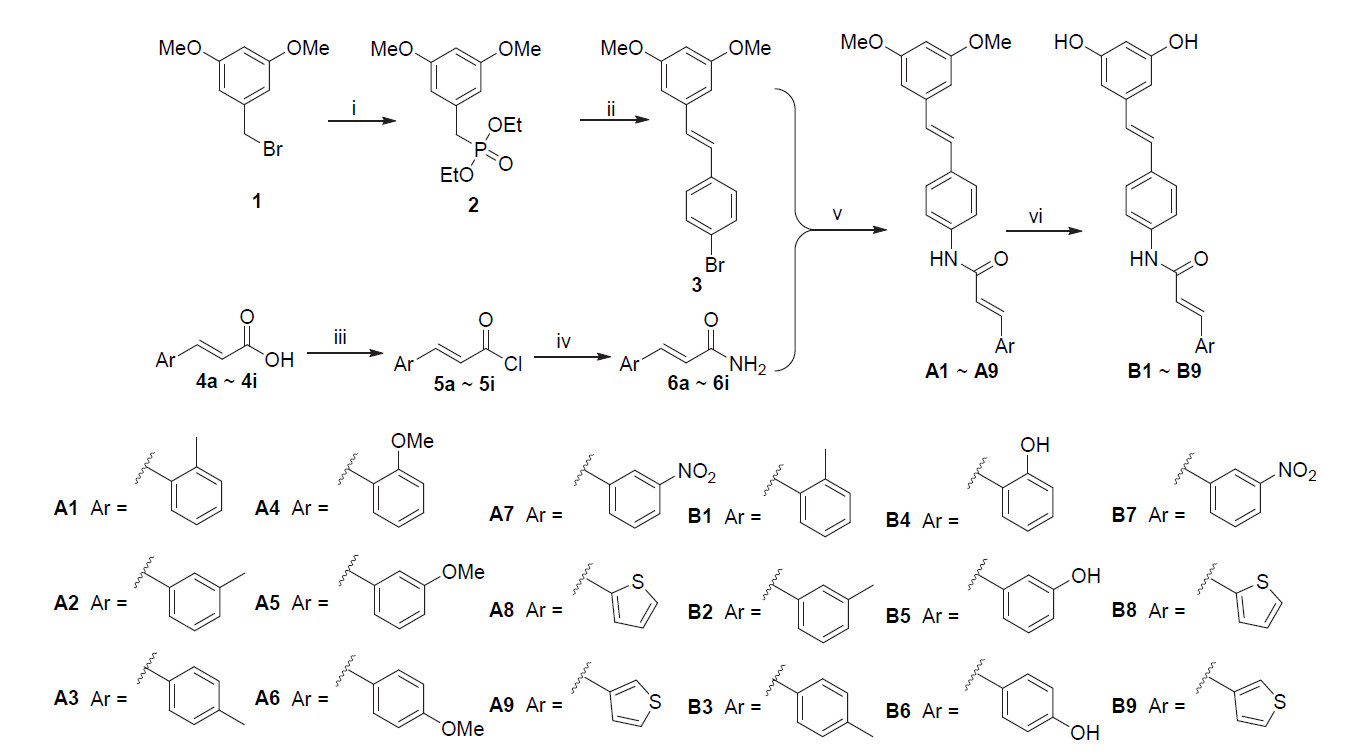
| Compd. | Ar | KG1/ (μmol•L–1) | MDA-MB-231/ (μmol•L–1) | Compd. | Ar | KG1/ (μmol•L–1) | MDA-MB-231/ (μmol•L–1) |
|---|---|---|---|---|---|---|---|
| A1 | o-Tolyl | 8.3 | 1.87 | B2 | m-Tolyl | 3.18 | 7.61 |
| A2 | m-Tolyl | >10 | >10 | B3 | p-Tolyl | 7.92 | 6.52 |
| A3 | p-Tolyl | >10 | >10 | B4 | 2-Hydroxyphenyl | 1.6 | 0.82 |
| A4 | 2-Methoxyphenyl | >10 | 3.91 | B5 | 3-Hydroxyphenyl | 3.03 | 1.53 |
| A5 | 3-Methoxyphenyl | >10 | 3.72 | B6 | 4-Hydroxyphenyl | 6.36 | 2.17 |
| A6 | 4-Methoxyphenyl | >10 | >10 | B7 | 3-Nitrophenyl | 2.58 | 3.49 |
| A7 | 3-Nitrophenyl | >10 | >10 | B8 | Thiophen-2-yl | 1.59 | 4.02 |
| A8 | Thiophen-2-yl | >10 | >10 | B9 | Thiophen-3-yl | 0.5 | 1.88 |
| A9 | Thiophen-3-yl | >10 | 2.7 | CL-2 | 1.35 | 2.90 | |
| B1 | o-Tolyl | 0.43 | 3.56 | VP16 | 0.91 | 6.62 |
| Compd. | Ar | KG1/ (μmol•L–1) | MDA-MB-231/ (μmol•L–1) | Compd. | Ar | KG1/ (μmol•L–1) | MDA-MB-231/ (μmol•L–1) |
|---|---|---|---|---|---|---|---|
| A1 | o-Tolyl | 8.3 | 1.87 | B2 | m-Tolyl | 3.18 | 7.61 |
| A2 | m-Tolyl | >10 | >10 | B3 | p-Tolyl | 7.92 | 6.52 |
| A3 | p-Tolyl | >10 | >10 | B4 | 2-Hydroxyphenyl | 1.6 | 0.82 |
| A4 | 2-Methoxyphenyl | >10 | 3.91 | B5 | 3-Hydroxyphenyl | 3.03 | 1.53 |
| A5 | 3-Methoxyphenyl | >10 | 3.72 | B6 | 4-Hydroxyphenyl | 6.36 | 2.17 |
| A6 | 4-Methoxyphenyl | >10 | >10 | B7 | 3-Nitrophenyl | 2.58 | 3.49 |
| A7 | 3-Nitrophenyl | >10 | >10 | B8 | Thiophen-2-yl | 1.59 | 4.02 |
| A8 | Thiophen-2-yl | >10 | >10 | B9 | Thiophen-3-yl | 0.5 | 1.88 |
| A9 | Thiophen-3-yl | >10 | 2.7 | CL-2 | 1.35 | 2.90 | |
| B1 | o-Tolyl | 0.43 | 3.56 | VP16 | 0.91 | 6.62 |
| [1] |
The International Agency for Research on Cancer (IARC), 2020. Available from https://www.iarc.fr/fr/news-events/latest-global-cancer-data-cancer-burden-rises-to-19-3-million-new-cases-and-10- 0-million-cancer-deaths-in-2020/.
|
| [2] |
Infante Lara, L.; Sledge, A.; Laradji, A.; Okoro, C. O.; Osheroff, N. Bioorg. Med. Chem. Lett. 2017, 27, 586.
doi: S0960-894X(16)31278-1 pmid: 27998679 |
| [3] |
Ortega, J. A.; Riccardi, L.; Minniti, E.; Borgogno, M.; Arencibia, J. M.; Greco, M. L.; Minarini, A.; Sissi, C.; De Vivo, M. J. Med. Chem. 2018, 61, 1375.
doi: 10.1021/acs.jmedchem.7b01388 |
| [4] |
Wang, Y.; Sun, H.; Xiao, Z.; Zhang, G.; Zhang, D.; Bao, X.; Li, F.; Wu, S.; Gao, Y.; Wei, N. Cell Commun. Signal 2018, 16, 52.
doi: 10.1186/s12964-018-0263-9 |
| [5] |
Heck, M. M.; Earnshaw, W. C. J. Cell Biol. 1986, 103, 2569.
pmid: 3025219 |
| [6] |
Lyu, Y. L.; Lin, C. P.; Azarova, A. M.; Cai, L.; Wang, J. C.; Liu, L. F. Mol. Cell Biol. 2006, 26, 7929.
doi: 10.1128/MCB.00617-06 |
| [7] |
Murty, M. S. R.; Penthala, R.; Polepalli, S.; Jain, N. Med. Chem. Res. 2016, 25, 627.
doi: 10.1007/s00044-016-1514-1 |
| [8] |
Smith, T. P.; Windsor, I. W.; Forest, K. T.; Raines, R. T. J. Med. Chem. 2017, 60, 7820.
doi: 10.1021/acs.jmedchem.7b00952 |
| [9] |
Xu, X. T.; Deng, X. Y.; Chen, J.; Liang, Q. M.; Zhang, K.; Li, D. L.; Wu, P. P.; Zheng, X.; Zhou, R. P.; Jiang, Z. Y.; Ma, A. J.; Chen, W. H.; Wang, S. H. Eur. J. Med. Chem. 2020, 189, 112013.
doi: 10.1016/j.ejmech.2019.112013 |
| [10] |
Harper, D. E.; Welch, D. R. J. Antibiot. (Tokyo) 1992, 45, 1827.
doi: 10.7164/antibiotics.45.1827 |
| [11] |
Sun, C. L.; Qu, P. P.; Li, F. Catal. Sci. Technol. 2014, 4, 988.
doi: 10.1039/C3CY00934C |
| [12] |
Yang, Y. H.; Li, G. H.; Song, Z. W.; Yang, X. L.; Liu, P. Lett. Org. Chem. 2010, 7, 533.
doi: 10.2174/157017810793362316 |
| [13] |
Hollywood, F.; Suschitzky, H.; Hull, R. Synthesis-Stuttgart 1982, 662.
|
| [14] |
Cai, M. Z.; Zhao, H.; Hu, W. Y. Chin. J. Chem. 2005, 23, 443.
doi: 10.1002/(ISSN)1614-7065 |
| [15] |
Maity, R.; Naskar, S.; Das, I. J. Org. Chem. 2018, 83, 2114.
doi: 10.1021/acs.joc.7b03054 |
| [16] |
Naskar, D.; Roy, S. J. Chem. Soc., Perkin Trans. 1 1999, 2435.
|
| [17] |
Feng, Z. X.; Zheng, Y. D.; Zhao, L.; Zhang, Z. Y.; Sun, Y.; Qiao, K.; Xie, Y. J.; Wang, Y. S.; He, W. Mater. Sci. Eng. C 2019, 104, 109944.
doi: 10.1016/j.msec.2019.109944 |
| [18] |
Mo, X. Y.; Wu, F. L.; Yu, B. L.; Wang, W. L.; Cai, X. L. Appl. Clay Sci. 2020, 193, 105664.
doi: 10.1016/j.clay.2020.105664 |
| [19] |
Shou, J. Q.; Wang, M.; Cheng, X. L.; Wang, X. Y.; Zhang, L. F.; Liu, Y. C.; Fei, C. Z.; Wang, C. M.; Gu, F.; Xue, F. Q.; Li, J.; Zhang, K. Y. Arch. Pharm. Res. 2020, 43, 271.
doi: 10.1007/s12272-020-01210-9 |
| [20] |
Chen, W. B.; Balakrishnan, K.; Kuang, Y. Y.; Han, Y. Y.; Fu, M.; Gandhi, V.; Peng, X. H. J. Med. Chem. 2014, 57, 4498.
doi: 10.1021/jm401349g |
| [21] |
Gootz, T. D.; Barrett, J. F.; Sutcliffe, J. A. Antimicrob. Agents Chemother. 1990, 34, 8.
doi: 10.1128/AAC.34.1.8 pmid: 2158274 |
| [22] |
Marini, J. C.; Levene, S. D.; Crothers, D. M.; Englund, P. T. Proc. Natl. Acad. Sci. Biol. 1982, 79, 7664.
doi: 10.1073/pnas.79.24.7664 |
| [23] |
Shen, Y.; Chen, W.; Li, Z. Y.; Shen, Y. M. Med. Chem. 2014, 10, 533.
pmid: 24047216 |
| [24] |
Shen, Y.; Chen, W.; Zhao, B. B.; Hao, H. L.; Li, Z. Y.; Lu, C. H.; Shen, Y. M. Biochem. Biophys. Res. Commun. 2014, 453, 302.
doi: 10.1016/j.bbrc.2014.09.042 |
| [25] |
Xiao, X.; Cushman, M. J. Am. Chem. Soc. 2005, 127, 9960.
doi: 10.1021/ja042485n |
| [26] |
Spitzer, R.; Jain, A. N. J. Comput. Aided Mol. Des. 2012, 26, 687.
doi: 10.1007/s10822-011-9533-y |
| [27] |
Morris, G. M.; Huey, R.; Lindstrom, W.; Sanner, M. F.; Belew, R. K.; Goodsell, D. S.; Olson, A. J. J. Comput. Chem. 2009, 30, 2785.
doi: 10.1002/jcc.v30:16 |
| [28] |
Sanner, M. F. J. Mol. Graphics Modell. 1999, 17, 57.
|
| [1] | Bozhen Wang, Jie Zhang, Chunhui Nian, Mingming Jin, Miaomiao Kong, Wulan Li, Wenfei He, Jianzhang Wu. Synthesis and Antitumor Activity of 3,4-Dichlorophenyl Amides [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 232-241. |
| [2] | Huanqing Li, Zhaohua Chen, Zujia Chen, Qiwen Qiu, Youcai Zhang, Sihong Chen, Zhaoyang Wang. Research Progress in Mercury Ion Fluorescence Probes Based on Organic Small Molecules [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3067-3077. |
| [3] | Panxing Pang, Rong Ning, Chuang Zhu, Wenjie Huang, Xianli Ma, Caina Jiang, Fangyao Li, Xiaoqun Zhou. Synthesis and in Vitro Antitumor Activity of Matrine Semicarbazide Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2126-2135. |
| [4] | Kanghui Duan, Junlong Tang, Wanqing Wu. Recent Advances in the Synthesis of Fused Heterocyclic Compounds and Their Antitumor Activities [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 826-854. |
| [5] | Weiqin Liu, Lihui Shao, Chengpeng Li, Yayu Zou, Haitao Long, Yan Li, Qiangsheng Ge, Zhenchao Wang, Guiping Ouyang. Synthesis and Antitumor Activity of 3-Hydrazone Quinazolinone Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 214-222. |
| [6] | Zhaohua Chen, Xiying Cao, Sihong Chen, Shiwei Yu, Yanlan Lin, Shuting Lin, Zhaoyang Wang. Design, Synthesis and Application of Trisubstituted Olefinic Aggregation-Induced Emission Molecules [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2355-2363. |
| [7] | Dongyan Hu, Guangtian Han, Xi'an Li, Huazhong Ren, Lirong Yue, Li Guo, Jiafu Feng. Synthesis and Evaluation in vitro of Novel Harmine Derivatives as Anticancer Activity Agents [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1863-1871. |
| [8] | Lu Xue, Lihua Zhang, Chengyu Zhang, Xin Zhao, Weifan Dang, Zhaoxin Wang, Chunhua Wang, Tongchuan Suo, Xiaohui Yan. Discovery of Tiancimycin Congeners from Streptomyces sp. CB03234-S [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1241-1247. |
| [9] | Honglin Dai, Xiaojie Si, Lingling Chi, Hao Wang, Chao Gao, Zhengjie Wang, Limin Liu, Jiajie Ma, Fuqiang Yu, Hongmin Liu, Yu Ke, Qiurong Zhang. Synthesis and Antitumor Activity Evaluation of 2,4,6-Trisubstituted Quinazoline Derivatives Containing Thiazole Structure [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3853-3862. |
| [10] | Zhengjie Wang, Honglin Dai, Xiaojie Si, Chao Gao, Limin Liu, Luye Zhang, Yang Zhang, Yadan Song, Peirong Zhao, Jiaxin Zheng, Yu Ke, Hongmin Liu, Qiurong Zhang. Synthesis and Antitumor Activity of 2,4,6-Trisubstituted Novel Quinazoline Derivatives Containing Trifluoromethyl [J]. Chinese Journal of Organic Chemistry, 2022, 42(1): 249-256. |
| [11] | Jiaoli Ma, Penghu Guo, Jing Li, Xincheng Liao, Huicheng Cheng. Synthesis and Antitumor Activity of Amide Derivatives Containing 1,3,4-Thiadiazole and Pyrazole Moieties [J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3214-3222. |
| [12] | Ningbo Li, Li Xu, Rong Ma, Qi Fan, Bo Li, Jie Qiao, Rui Guo, Xinhua Xu. Synthesis and Antitumor Activities of Novel Organic Sulfur (Selenium) Tegafur Derivatives [J]. Chinese Journal of Organic Chemistry, 2021, 41(7): 2723-2734. |
| [13] | Yinghao Liu, Fangxia Lin, Yinfeng Tan, Jingyu Yang, Bin Zhang, Xueming Zhou, Xinming Song. Three New Phenanthraquinones from the Root of Dendrobium nobile [J]. Chinese Journal of Organic Chemistry, 2021, 41(5): 2112-2115. |
| [14] | Yuxun Zhao, Yunyun Wang, Chenglong Zhang, Xu Xu, Shifa Wang. Synthesis of Novel Camphor Sulfamoxime Ether Derivatives and Its Application in Antitumor Activity [J]. Chinese Journal of Organic Chemistry, 2021, 41(3): 1224-1233. |
| [15] | Jing Cong, Fang Fang, Liangmin Xue, Meng Wang, Chao Tian, Xiaowei Wang, Junyi Liu, Zhili Zhang. Synthesis and Antitumor Activity of Novel Pyrimidine Monocyclic Nonclassical Antifolates [J]. Chinese Journal of Organic Chemistry, 2021, 41(2): 776-787. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||