Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (7): 2155-2163.DOI: 10.6023/cjoc202201036 Previous Articles Next Articles
ARTICLES
陈学荣a, 祁亮a, 黄晋培a, 朱伟伟b,*( ), 周益峰a,*(
), 周益峰a,*( )
)
收稿日期:2022-01-22
修回日期:2022-02-17
发布日期:2022-08-09
通讯作者:
朱伟伟, 周益峰
作者简介:基金资助:
Xuerong Chena, Liang Qia, Jinpei Huanga, Weiwei Zhub( ), Yifeng Zhoua(
), Yifeng Zhoua( )
)
Received:2022-01-22
Revised:2022-02-17
Published:2022-08-09
Contact:
Weiwei Zhu, Yifeng Zhou
About author:Supported by:Share
Xuerong Chen, Liang Qi, Jinpei Huang, Weiwei Zhu, Yifeng Zhou. Lewis Acid Promoted Intramolecular Nucleophilic Heterocyclization of Unsaturated Amides for the Synthesis of 2-Oxazolines and Their Applications in Imaging of Living Cells[J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2155-2163.
| Entry | LAb | Solvent | Temperature/℃ | Yieldc/% |
|---|---|---|---|---|
| 1 | BF3•Et2O | DCM | 0 | 12 |
| 2 | BF3•Et2O | DCM | 25 | 65 |
| 3 | TFA | DCM | 25 | 0 |
| 4 | AcOH | DCM | 25 | 0 |
| 5 | MsOH | DCM | 25 | 0 |
| 6 | FeCl3 | DCM | 25 | 0 |
| 7 | ZnCl2 | DCM | 25 | 0 |
| 8 | MeOTf | DCM | 25 | 0 |
| 9 | TMSOTf | DCM | 25 | 85 |
| 10 | TMSOTf | MeCN | 25 | 82 |
| 11 | TMSOTf | C6H5Cl | 25 | 79 |
| 12 | TMSOTf | CHCl3 | 25 | 77 |
| 13 | TMSOTf | DCE | 25 | 94 |
| 14 | TMSOTf | DMF | 25 | Trace |
| 15d | TMSOTf | DCE | 25 | Trace |
| Entry | LAb | Solvent | Temperature/℃ | Yieldc/% |
|---|---|---|---|---|
| 1 | BF3•Et2O | DCM | 0 | 12 |
| 2 | BF3•Et2O | DCM | 25 | 65 |
| 3 | TFA | DCM | 25 | 0 |
| 4 | AcOH | DCM | 25 | 0 |
| 5 | MsOH | DCM | 25 | 0 |
| 6 | FeCl3 | DCM | 25 | 0 |
| 7 | ZnCl2 | DCM | 25 | 0 |
| 8 | MeOTf | DCM | 25 | 0 |
| 9 | TMSOTf | DCM | 25 | 85 |
| 10 | TMSOTf | MeCN | 25 | 82 |
| 11 | TMSOTf | C6H5Cl | 25 | 79 |
| 12 | TMSOTf | CHCl3 | 25 | 77 |
| 13 | TMSOTf | DCE | 25 | 94 |
| 14 | TMSOTf | DMF | 25 | Trace |
| 15d | TMSOTf | DCE | 25 | Trace |
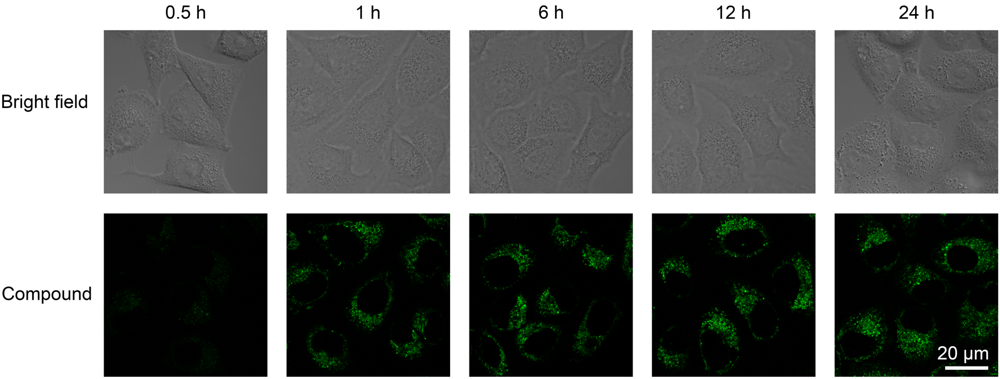
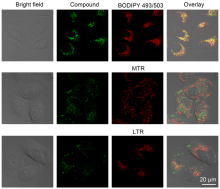

| [1] |
(a) Taylor, A. P.; Robinson, R. P.; Fobian, Y. M.; Blakemore, D. C.; Jones, L. H.; Fadeyi, O. Org. Biomol. Chem. 2016, 14, 6611.
doi: 10.1039/C6OB00936K pmid: 5723975 |
|
(b) Pawlowski, R.; Stanek, F.; Stodulski, M. Molecules 2019, 24, 1533.
doi: 10.3390/molecules24081533 pmid: 5723975 |
|
|
(c) Luo, Y.; Li, B.; Wang, W.; Wu, K.; Tan, B. Adv. Mater. 2012, 24, 5703.
doi: 10.1002/adma.201202447 pmid: 5723975 |
|
|
(d) Ryu, D.; Mouchiroud, L.; Andreux, P. A.; Katsyuba, E.; Moullan, N.; Felix, A. A. N.; Williams, E. G.; Jha, P.; Lo Sasso, G.; Huzard, D.; Aebischer, P.; Sandi, C.; Rinsch, C.; Auwerx, J. Nat. Med. 2016, 22, 879.
doi: 10.1038/nm.4132 pmid: 5723975 |
|
|
(e) Gandini, A.; Lacerda, T. M.; Carvalho, A. J. F.; Trovatti, E. Chem. Rev. 2016, 116, 1637.
doi: 10.1021/acs.chemrev.5b00264 pmid: 5723975 |
|
|
(f) Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Nat. Chem. 2016, 8, 531.
doi: 10.1038/nchem.2479 pmid: 5723975 |
|
|
(g) Boiani, M.; González, M. Mini Rev. Med. Chem. 2005, 5, 409.
doi: 10.2174/1389557053544047 pmid: 5723975 |
|
|
(h) Bignan, G. C.; Battista, K.; Connolly, P. J.; Orsini, M. J.; Liu, J.; Middleton, S. A.; Reitz, A. B. Bioorg. Med. Chem. Lett. 2005, 15, 5022.
doi: 10.1016/j.bmcl.2005.08.009 pmid: 5723975 |
|
|
(i) Yurchenko, R. I.; Ponomarenko, A. D.; Svarovskaya, N. N. Chem. Heterocycl. Compd. 2005, 41, 656.
doi: 10.1007/s10593-005-0198-0 pmid: 5723975 |
|
|
(j) Hecker, E. Cancer Res. 1968, 28, 2338.
pmid: 5723975 |
|
|
(k) Patil, S. A.; Patil, S. A.; Patil, R.; Keri, R. S.; Budagumpi, S.; Balakrishna, G. R.; Tacke, M. Future Med. Chem. 2015, 7, 1305.
doi: 10.4155/fmc.15.61 pmid: 5723975 |
|
|
(l) Yang, B.; Zhao, M.; Fang, X.; Yang, X. Y.; Wu, H. F. Chin. J. Org. Chem. 2013, 33, 1088. (in Chinese)
doi: 10.6023/cjoc201210034 pmid: 5723975 |
|
|
( 杨波, 赵敏, 方向, 杨雪艳, 吴范宏, 有机化学, 2013, 33, 1088.)
doi: 10.6023/cjoc201210034 pmid: 5723975 |
|
| [2] |
(a) Kawai, H.; Shibata, N. Chem. Rec. 2014, 14, 1024.
doi: 10.1002/tcr.201402023 pmid: 19378971 |
|
(b) Hargaden, G. C.; Guiry, P. J. Chem. Rev. 2009, 109, 2505.
doi: 10.1021/cr800400z pmid: 19378971 |
|
|
(c) Li, Q.; Woods, K. W.; Claiborne, A.; Gwaltney, N. S.; Barr, K. J.; Liu, G.; Gehrke, L.; Credo, R. B.; Hui, Y. H.; Lee, J. Bioorg. Med. Chem. Lett. 2010, 33, 465.
pmid: 19378971 |
|
|
(d) Dahiya, S.; Dahiya, R. Eur. J. Med. Chem. 2021, 218, 113406.
doi: 10.1016/j.ejmech.2021.113406 pmid: 19378971 |
|
|
(e) Moraski, G. C.; Chang, M.; Estrada, A. V.; Franzblau, S. G.; Möllmann, U.; Miller, M. J. Eur. J. Med. Chem. 2010, 45, 1703.
doi: 10.1016/j.ejmech.2009.12.074 pmid: 19378971 |
|
| [3] |
(a) Verbraeken, B.; Hullaert, J.; Guyse, J. V.; Hecke, K. V.; Winne, J.; Hoogenboom, R. J. Am. Chem. Soc. 2018, 140, 17404.
doi: 10.1021/jacs.8b10918 pmid: 30501590 |
|
(b) Chiacchio, M. A.; Lanza, G.; Chiacchio, U.; Giofrè, S. V.; Romeo, R.; Iannazzo, D.; Legnani, L. Curr. Med. Chem. 2019, 26, 7337.
doi: 10.2174/0929867326666181203130402 pmid: 30501590 |
|
|
(c) Zhang, H. Z.; Zhao, Z. L.; Zhou, C. H. Eur. J. Med. Chem. 2017, 12, 044.
pmid: 30501590 |
|
| [4] |
(a) Connon, R.; Roche, B.; Rokade, B. V.; Guiry, P. J. Chem. Rev. 2021, 121, 6373.
doi: 10.1021/acs.chemrev.0c00844 pmid: 12007099 |
|
(b) Rao, B. V.; Bhaskar, G.; Kumar, V. S. Tetrahedron: Asymmetry 2004, 15, 1279.
pmid: 12007099 |
|
|
(c) Thorhauge, J.; Roberson, M.; Hazell, R. G.; Jørgensen, K. A. Chem.-Eur. J. 2002, 8, 1888.
pmid: 12007099 |
|
| [5] |
(a) Hayes, G.; Drain, B.; Becer, R. Macromolecules 2022, 55, 146.
doi: 10.1021/acs.macromol.1c02245 |
|
(b) Trachsel, L.; Wong, M. Z.; Benetti, E. M. Biomater. Sci. 2021, 9, 2874.
doi: 10.1039/D0BM02217A |
|
|
(c) Sedlacek, O.; Monnery, B. D.; Filippov, S. K.; Hoogenboom, R.; Hruby, M. Macromol. Rapid Commun. 2012, 33, 1648.
doi: 10.1002/marc.201200453 |
|
|
(d) Simon, L.; Marcotte, N.; Devoisselle, J. M.; Begu, S.; Lapinte, V. Int. J. Pharm. 2020, 585, 119536.
doi: 10.1016/j.ijpharm.2020.119536 |
|
|
(e) Luxenhofer, R.; Han, Y. C.; Schulz, A.; Tong, J.; He, Z.J.; Kabanov, A. V.; Jordan, R. Macromol. Rapid Commun. 2012, 33, 1613.
doi: 10.1002/marc.201200354 |
|
| [6] |
(a) Helmut, W.; Wolfgang, S. Angew. Chem., Int. Ed. 1972, 11, 287.
pmid: 25742052 |
|
(b) Alhalib, A.; Kamouka, S.; Moran, W. J. Org. Lett. 2015, 17, 1453.
doi: 10.1021/acs.orglett.5b00333 pmid: 25742052 |
|
|
(c) Li, B.; Wang, S. Q.; Liu, B.; Shi, B. F. Org. Lett. 2015, 17, 1200.
doi: 10.1021/acs.orglett.5b00151 pmid: 25742052 |
|
|
(d) Liu, G. Q.; Yang, C. H.; Li, Y. M. J. Org. Chem. 2015, 80, 11339.
doi: 10.1021/acs.joc.5b01832 pmid: 25742052 |
|
| [7] |
(a) Mulahmetovic, E.; Hargaden, G. C. Mini Rev. Org. Chem. 2019, 16, 507.
doi: 10.2174/1570193X15666180802105505 |
|
(b) Ilkgul, B.; Gunes, D.; Sirkecioglu, O.; Bicak, N. Tetrahedron Lett. 2010, 51, 5313.
doi: 10.1016/j.tetlet.2010.07.167 |
|
|
(c) Goud, D. R.; Pathak, U. Synthesis 2012, 44, 3678.
doi: 10.1055/s-0032-1317341 |
|
|
(d) Brandstätter, M.; Roth, F.; Luedtke, N. W. J. Org. Chem. 2015, 80, 40.
doi: 10.1021/jo5016695 |
|
|
(e) Chen, Z. G.; Hou, D.; Liu, D. E.; Hui, W. P. Chin. J. Org. Chem. 2016, 36, 2191. (in Chinese)
doi: 10.6023/cjoc201603032 |
|
|
( 陈战国, 侯丹, 刘德娥, 惠文萍, 有机化学, 2016, 36, 2191.)
doi: 10.6023/cjoc201603032 |
|
|
(f) Wu, F.; Kaur, N.; Alom, N. E.; Li, W. JACS Au 2021, 1, 734.
doi: 10.1021/jacsau.1c00103 |
|
| [8] |
(a) Zhou, M. N.; Delaveris, C. S.; Kramer, J. R.; Kenkel, J. A.; Engleman, E. G.; Bertozzi, C. R. Angew. Chem., Int. Ed. 2018, 57, 3137.
doi: 10.1002/anie.201713075 |
|
(b) Mizukami, S.; Hori, Y.; Kikuchi, K. Acc. Chem. Res. 2014, 47, 247.
doi: 10.1021/ar400135f |
|
|
(c) Li, B.; Zhou, X. H.; Yang, P. Y.; Zhu, L. P.; Zhong, Y.; Cai, Z. J.; Jiang, B.; Cai, X. Q.; Jiang, X. X. Adv. Sci. 2019, 6, 1802039.
doi: 10.1002/advs.201802039 |
|
| [9] |
(a) Janzen, W. P. Chem. Biol. 2014, 21, 1162.
doi: 10.1016/j.chembiol.2014.07.015 |
|
(b) Liu, L. Y.; Liu, W. T.; Wang, K. N.; Zhu, B. C.; Xia, X. Y.; Ji, L. N.; Mao, Z. W. Angew. Chem., Int. Ed. 2020, 59, 9719.
doi: 10.1002/anie.202002422 |
|
| [10] |
(a) Maity, A.; Teets, T. S. Chem. Rev. 2016, 116, 8873.
doi: 10.1021/acs.chemrev.6b00034 pmid: 27164024 |
|
(b) North, M.; Usanov, D. L.; Young, C. Chem. Rev. 2008, 108, 5146.
pmid: 27164024 |
|
|
(c) Hong, M.; Chen, J.; Chen, E. Y. X. Chem. Rev. 2018, 118, 10551.
doi: 10.1021/acs.chemrev.8b00352 pmid: 27164024 |
|
| [11] |
(a) Zhang, X.; Wan, X. T.; Cong, Y.; Zhen, X. H.; Li, Q.; Negrerie, D. Z.; Du, Y. F.; Zhao, K. J. Org. Chem. 2019, 84, 10402.
doi: 10.1021/acs.joc.9b01601 |
|
(b) Nakao, Y.; Idei, H.; Kanyiva, K. S.; Hiyama, T. J. Am. Chem. Soc. 2009, 131, 5070.
doi: 10.1021/ja901153s |
|
|
(c) An, J. K.; Liu, J. K.; Shi, Y.; Zhu, W. W.; Guo, G. Y.; Jiang, X. X.; Xue, J. J.; Zhang, H. R. Curr. Org. Chem. 2020, 24, 1263.
doi: 10.2174/1385272824999200701120327 |
|
|
(d) Gao, W. C.; Liu, T.; Zhang, B.; Li, X.; Wei, W. L.; Liu, Q.; Tian, J.; Chang, H. H. J. Org. Chem. 2016, 81, 11297.
doi: 10.1021/acs.joc.6b02271 |
|
| [12] |
Santos, F. S.; Zanotto, G. M.; Argomedo, L. M. Z.; Darben, M. P.; Goncalves, P. F. B.; Stefani, H. A.; Rodembusch, F. S. Dyes Pigm. 2019, 165, 372.
doi: 10.1016/j.dyepig.2019.02.047 |
| [1] | Zujia Chen, Shiwei Yu, Yongjun Zhou, Huanqing Li, Qiwen Qiu, Miaoxin Li, Zhaoyang Wang. Application of BF3•OEt2 in Organic Synthesis as a Catalyst or Synthon [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3107-3118. |
| [2] | Yifang Li, Yao Wang, Huawei Niu, Xiujin Chen, Zhaozhou Li, Yongguo Wang. Research Progress of Sulfur Dioxide Fluorescent Probe Targeting Mitochondria [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1952-1962. |
| [3] | Yang Zhao, Panpan Chen, Gaonan Li, Zhigang Niu, Enju Wang. Tetraarylimidazole-Based Aggregation-Induced Emission Luminogens and Their Cell-Imaging Application [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2156-2162. |
| [4] | Weidi Cao, Xiaohua Liu. Recent Advances on Catalytic Enantioselective Protonation for Construction of α-Tertiary Carbonyl Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 961-973. |
| [5] | Hongwei Tang, Chao Wang, Keli Zhong, Shuhua Hou, Lijun Tang, Yanjiang Bian. A Naked-Eye and Fluorescent Dual-Channel Probe for Rapid Detection of Hg2+ and Its Multiple Applications [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 712-717. |
| [6] | Yiling Zhao, Zhikang Chen, Lei Li, Conglei Liu, Hongping Zhu. Silylene/Organoaluminum Lewis Pair System and the Initiation Property for Polymerization of (Meth)acrylates [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3590-3597. |
| [7] | Xiangqing Feng, Haifeng Du. B(C6F5)3-Catalyzed Silylation of Unsaturated Hydrocarbons [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3544-3557. |
| [8] | Meng Liu, Yanru Huang, Xiaofei Sun, Lijun Tang. An “Aggregation-Induced Emission+Excited-State Intramolecular Proton Transfer” Mechanisms-Based Benzothiazole Derived Fluorescent Probe and Its ClO– Recognition [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 345-351. |
| [9] | Dan Ba, Guolin Cheng. Blue Light Induced Insertion of Carbene into C(CO)—C Bonds of 1,3-Diones [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2888-2897. |
| [10] | Jiaxin Li, Ruyan He, Senlin Duan, Jinhua Li, Xiaojing Han, Yong Ye. Construction and Cell Imaging Study of a Novel Fluorescent Probe for ONOO– Detection [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2428-2432. |
| [11] | Xiuliang Cheng, Dong Li, Boxuan Yang, Yumei Lin, Lei Gong. Recent Advances in Visible-Light Photocatalytic Asymmetric Synthesis Enabled by Chiral Lewis Acids [J]. Chinese Journal of Organic Chemistry, 2022, 42(10): 3335-3350. |
| [12] | Chao Wang, Xin Wang, Keli Zhong, Shuhua Hou, Xiaomei Yan, Yanjiang Bian, Lijun Tang. A Long-Wavelength Fluorescent Probe for Naked Eye Recognition of HSO3-/SO32- in Aqueous Solution and Its Application [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2417-2423. |
| [13] | Keli Zhong, Lulu Zhou, Lin Chen, Lijun Tang, Xue Gao, Xiuying Liu, Xiuxiu Pang, Xiaomei Yan. Synthesis of 2-(3-Cyanofuran-2(5H)-ylidene)malononitrile Derivative and Its Recognition for Pd2+ [J]. Chinese Journal of Organic Chemistry, 2021, 41(3): 1124-1130. |
| [14] | Yang Min, Xia Lili, Zhou Xiaoqin, Jia Chengli, Ji Min, Wang Peng. A Ratiometric Fluorescent Probe for Imaging Hydrogen Peroxide in Living Cells [J]. Chinese Journal of Organic Chemistry, 2020, 40(9): 2888-2894. |
| [15] | Mei Huihui, Yang Mingyang, Liu Xiaoyan, Tian Yuping, Xu Kuoxi. A Novel Phenanthroline-Based Fluorescent Probe for Pb(II) [J]. Chinese Journal of Organic Chemistry, 2020, 40(8): 2508-2512. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||