Chinese Journal of Organic Chemistry ›› 2025, Vol. 45 ›› Issue (12): 4490-4496.DOI: 10.6023/cjoc202503031 Previous Articles Next Articles
ARTICLES
收稿日期:2025-03-31
修回日期:2025-05-08
发布日期:2025-06-05
通讯作者:
陈洁, 王斌
基金资助:
Bo Cao, Xiang Wen, Jie Chen*( ), Bin Wang*(
), Bin Wang*( )
)
Received:2025-03-31
Revised:2025-05-08
Published:2025-06-05
Contact:
Jie Chen, Bin Wang
Supported by:Share
Bo Cao, Xiang Wen, Jie Chen, Bin Wang. “Click” Tetradentate Nitrogen Donor Ligands for Nonheme Iron-Catalyzed Asymmetric Epoxidation Reactions[J]. Chinese Journal of Organic Chemistry, 2025, 45(12): 4490-4496.
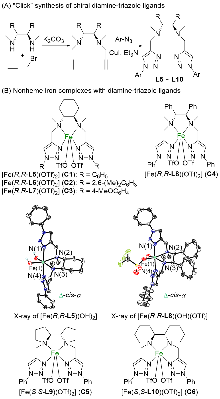
| Entry | Cat. | Temp./℃ | Acid | Yield b/% | ee c/% |
|---|---|---|---|---|---|
| 1 | C1 | r.t. | HOAc | 93 | 75 |
| 2 | C2 | r.t. | HOAc | 76 | 57 |
| 3 | C3 | r.t. | HOAc | 81 | 61 |
| 4 | C4 | r.t. | HOAc | 84 | 64 |
| 5 | C5 | r.t. | HOAc | 86 | 72 |
| 6 | C6 | r.t. | HOAc | 85 | 70 |
| 7 | C1 | 0 | HOAc | 98 | 80 |
| 8 | C1 | -20 | HOAc | 99 | 82 |
| 9 | C1 | -30 | HOAc | 99 | 84 |
| 10d | C1 | -30 | eba | 99 | 91 |
| 11d | C1 | -30 | 2-eha | 99 | 86 |
| 12d | C1 | -30 | cxa | 99 | 73 |
| 13d | C1 | -30 | dmba | 78 | 73 |
| 14d | C1 | -30 | aca | 81 | 68 |
| 15d | C1 | -30 | (S)-2-mba | 25 | 56 |
| 16 | C1 | -30 | (S)-Ibuprofen | 54 | 73 |
| Entry | Cat. | Temp./℃ | Acid | Yield b/% | ee c/% |
|---|---|---|---|---|---|
| 1 | C1 | r.t. | HOAc | 93 | 75 |
| 2 | C2 | r.t. | HOAc | 76 | 57 |
| 3 | C3 | r.t. | HOAc | 81 | 61 |
| 4 | C4 | r.t. | HOAc | 84 | 64 |
| 5 | C5 | r.t. | HOAc | 86 | 72 |
| 6 | C6 | r.t. | HOAc | 85 | 70 |
| 7 | C1 | 0 | HOAc | 98 | 80 |
| 8 | C1 | -20 | HOAc | 99 | 82 |
| 9 | C1 | -30 | HOAc | 99 | 84 |
| 10d | C1 | -30 | eba | 99 | 91 |
| 11d | C1 | -30 | 2-eha | 99 | 86 |
| 12d | C1 | -30 | cxa | 99 | 73 |
| 13d | C1 | -30 | dmba | 78 | 73 |
| 14d | C1 | -30 | aca | 81 | 68 |
| 15d | C1 | -30 | (S)-2-mba | 25 | 56 |
| 16 | C1 | -30 | (S)-Ibuprofen | 54 | 73 |
| Entry | Substrate | Yieldb/% | eec/% |
|---|---|---|---|
| | |||
| 1 | 1a: R1=R2=C6H5 | 99 | 91 |
| 2 | 2a: R1=p-MeC6H4, R2=C6H5 | 99 | 94 |
| 3 | 3a: R1=p-FC6H4, R2=C6H5 | 99 | 93 |
| 4 | 4a: R1=p-ClC6H4, R2=C6H5 | 99 | 92 |
| 5 | 5a: R1=p-BrC6H4, R2=C6H5 | 99 | 93 |
| 6 | 6a: R1=C6H5, R2=p-MeOC6H4 | 96 | 82 |
| 7 | 7a: R1=C6H5, R2=p-MeC6H4 | 98 | 85 |
| 8 | 8a: R1=C6H5, R2=p-FC6H4 | 99 | 91 |
| 9 | 9a: R1=C6H5, R2=p-ClC6H4 | 99 | 90 |
| 10 | 10a: R1=C6H5, R2=p-BrC6H4 | 99 | 90 |
| 11 | 11a: R1=C6H5, R2=2-naphthyl | 94 | 83 |
| 12 | | 89 | 87 |
| 13 | | 95 | 89 |
| 14 | | 82 | 52 |
| 15 | | 92 | 66 |
| 16 | | 75 | 19 |
| | |||
| 17 | 17a: R1=C6H5, R2=Me | 99 | 87 |
| 18 | 18a: R1=C6H5, R2=Et | 99 | 90 |
| 19 | 19a: R1=o-ClC6H4, R2=Et | 99 | 92 |
| 20 | 20a: R1=m-ClC6H4, R2=Et | 99 | 91 |
| 21 | 21a: R1=p-ClC6H4, R2=Et | 99 | 93 |
| 22 | 22a: R1=p-BrC6H4, R2=Et | 99 | 92 |
| Entry | Substrate | Yieldb/% | eec/% |
|---|---|---|---|
| | |||
| 1 | 1a: R1=R2=C6H5 | 99 | 91 |
| 2 | 2a: R1=p-MeC6H4, R2=C6H5 | 99 | 94 |
| 3 | 3a: R1=p-FC6H4, R2=C6H5 | 99 | 93 |
| 4 | 4a: R1=p-ClC6H4, R2=C6H5 | 99 | 92 |
| 5 | 5a: R1=p-BrC6H4, R2=C6H5 | 99 | 93 |
| 6 | 6a: R1=C6H5, R2=p-MeOC6H4 | 96 | 82 |
| 7 | 7a: R1=C6H5, R2=p-MeC6H4 | 98 | 85 |
| 8 | 8a: R1=C6H5, R2=p-FC6H4 | 99 | 91 |
| 9 | 9a: R1=C6H5, R2=p-ClC6H4 | 99 | 90 |
| 10 | 10a: R1=C6H5, R2=p-BrC6H4 | 99 | 90 |
| 11 | 11a: R1=C6H5, R2=2-naphthyl | 94 | 83 |
| 12 | | 89 | 87 |
| 13 | | 95 | 89 |
| 14 | | 82 | 52 |
| 15 | | 92 | 66 |
| 16 | | 75 | 19 |
| | |||
| 17 | 17a: R1=C6H5, R2=Me | 99 | 87 |
| 18 | 18a: R1=C6H5, R2=Et | 99 | 90 |
| 19 | 19a: R1=o-ClC6H4, R2=Et | 99 | 92 |
| 20 | 20a: R1=m-ClC6H4, R2=Et | 99 | 91 |
| 21 | 21a: R1=p-ClC6H4, R2=Et | 99 | 93 |
| 22 | 22a: R1=p-BrC6H4, R2=Et | 99 | 92 |
| [10] |
(p)
doi: 10.1021/cs300205n pmid: 31881152 |
| [11] |
(a)
doi: 10.1002/chem.201103802 pmid: 22581474 |
|
(b)
doi: 10.1002/chem.201200992 pmid: 22581474 |
|
|
(c)
doi: 10.1021/acscatal.7b03601 pmid: 22581474 |
|
|
(d)
doi: 10.1039/D0CC04440G pmid: 22581474 |
|
|
(e)
doi: 10.1002/adsc.v359.15 pmid: 22581474 |
|
|
(f)
doi: 10.1021/jacs.3c06231 pmid: 22581474 |
|
| [12] |
(a)
doi: 10.1021/ol401812h |
|
(b)
doi: 10.1021/acs.orglett.5b00018 |
|
|
(c)
doi: 10.1021/ol402612x |
|
|
(d)
doi: 10.1021/acscatal.7b00968 |
|
| [13] |
doi: 10.1002/(ISSN)1521-3773 |
| [14] |
(a)
doi: 10.1002/(ISSN)1521-3773 |
|
(b)
doi: 10.1021/jo011148j |
|
|
(c)
doi: 10.1021/om700669v |
|
|
(d)
doi: 10.1021/ja072679d |
|
| [15] |
doi: 10.1039/b922043g |
| [16] |
doi: 10.1021/acs.chemrev.2c00724 |
| [17] |
(a)
doi: 10.31635/ccschem.022.202201780 |
| [1] |
|
| [2] |
(a)
doi: 10.1021/cr0306945 pmid: 15884785 |
|
(b)
pmid: 15884785 |
|
|
(c)
doi: 10.1021/cr068367v pmid: 15884785 |
|
|
(d)
doi: 10.1039/C0CS00077A pmid: 15884785 |
|
|
(e)
doi: 10.1002/anie.v53.29 pmid: 15884785 |
|
|
(f)
doi: 10.1021/acs.chemrev.7b00167 pmid: 15884785 |
|
| [17] |
(b)
doi: 10.1021/ja9638521 |
| [18] |
doi: 10.1021/ja201873d pmid: 21553923 |
| [19] |
pmid: 11151391 |
| [2] |
(g)
doi: 10.1016/j.ccr.2021.214065 pmid: 15884785 |
|
(h)
doi: 10.1021/jacs.6b10836 pmid: 15884785 |
|
| [3] |
doi: 10.1021/acs.chemrev.7b00373 |
| [4] |
doi: 10.1007/s00775-016-1431-2 |
| [5] |
(a)
doi: 10.1021/acs.accounts.9b00285 |
|
(b)
doi: 10.1021/acs.accounts.5b00053 |
|
|
(c)
doi: 10.1021/acscatal.0c02073 |
|
|
(d)
doi: 10.1039/C5CC05576H |
|
|
(e)
doi: 10.1002/ejoc.v27.40 |
|
|
(f)
doi: 10.1016/j.ccr.2012.04.005 |
|
|
(g)
doi: 10.1016/j.ccr.2020.213443 |
|
|
(h)
doi: 10.1007/s11426-023-1578-y |
|
| [6] |
(a)
doi: 10.1002/tcr.v21.12 |
|
(b)
doi: 10.1007/s00775-016-1434-z |
|
|
(c)
doi: 10.1039/D0CS00340A |
|
|
(d)
doi: 10.1021/acscatal.3c02282 |
|
|
(e)
doi: 10.6023/cjoc202006008 |
|
|
(孙强盛, 孙伟, 有机化学, 2020, 40, 3686.)
doi: 10.6023/cjoc202006008 |
|
| [7] |
(a)
doi: 10.1038/s41570-020-0197-9 |
|
(b)
doi: 10.1016/j.ccr.2019.01.010 |
|
|
(c)
doi: 10.1016/j.ccr.2024.216429 |
|
| [8] |
(a)
doi: 10.1002/chem.201905119 pmid: 31804749 |
|
(b)
doi: 10.1021/acscatal.1c01993 pmid: 31804749 |
|
| [9] |
(a)
pmid: 11459514 |
|
(b)
doi: 10.1021/ja029962r pmid: 11459514 |
|
|
(c)
doi: 10.1126/science.1148597 pmid: 11459514 |
|
| [10] |
(a)
doi: 10.1021/jacs.5b11579 pmid: 31881152 |
|
(b)
doi: 10.1021/acscatal.0c02634 pmid: 31881152 |
|
|
(c)
doi: 10.1021/ol901400m pmid: 31881152 |
|
|
(d)
doi: 10.1021/acscatal.8b04463 pmid: 31881152 |
|
|
(e)
doi: 10.1021/ja4078446 pmid: 31881152 |
|
|
(f)
doi: 10.1002/anie.201410557 pmid: 31881152 |
|
|
(g)
doi: 10.1021/acscentsci.6b00368 pmid: 31881152 |
|
|
(h)
doi: 10.1021/jacs.2c10148 pmid: 31881152 |
|
|
(i)
doi: 10.1021/jacs.9b12239 pmid: 31881152 |
|
|
(j)
doi: 10.1021/acscatal.3c04832 pmid: 31881152 |
|
|
(k)
doi: 10.1021/acscatal.1c00811 pmid: 31881152 |
|
|
(l)
doi: 10.1021/acscatal.6b02851 pmid: 31881152 |
|
|
(m)
doi: 10.1021/acscatal.6b01473 pmid: 31881152 |
|
|
(n)
doi: 10.1021/acscatal.5b02299 pmid: 31881152 |
|
|
(o)
doi: 10.1021/cs500333c pmid: 31881152 |
| [1] | Ye Wu, Chunxia Chen, Jinsong Peng. Research Progress of C—H Bond Asymmetric Hydroxylation [J]. Chinese Journal of Organic Chemistry, 2025, 45(8): 2726-2745. |
| [2] | Zhe Han, Yin Cui, Chengrong Ding, Jian Yang, Guofu Zhang. Research Progress on the Synthetic Applications of Direct Construction of Sulfonyl Fluoride Functional Groups Using "SO2F" Sources [J]. Chinese Journal of Organic Chemistry, 2025, 45(7): 2245-2264. |
| [3] | Jing Leng, Hao Huang, Jie Xu, Huali Qin. Research Progress on the Synthesis of Sulfonyl Fluoride Compounds Based on Small Molecule Sulfur-Fluoride Building Blocks [J]. Chinese Journal of Organic Chemistry, 2025, 45(4): 1223-1238. |
| [4] | Jia Zhao, Qiuyun Gan, Yaofeng Yuan. Research Progress in Free Radical Fluorosulfonylation [J]. Chinese Journal of Organic Chemistry, 2025, 45(4): 1206-1222. |
| [5] | Xin Liu, Shixin Zheng, Huajie Zhao, Jie Chen, Bin Wang. Recent Advances in Biomimetic Asymmetric Catalysis for Olefin cis-Dihydroxylation [J]. Chinese Journal of Organic Chemistry, 2025, 45(12): 4257-4270. |
| [6] | Yadan Chen, Songgang Huang, Jie Chen, Bin Wang. Nonheme Iron(III)-Monoamidate Complexes as Catalysts for Methylene-Selective Oxidation of Simple Alkanes [J]. Chinese Journal of Organic Chemistry, 2025, 45(12): 4453-4462. |
| [7] | Hao Zhang, Qingbin Zhao, Zhongrui Ruan, Zhenxing Liu. Recent Development of SuFEx Reaction between Silyl Ethers and Sulfur(VI) Fluoride Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3569-3579. |
| [8] | Biao Han, Yaoyao Zhang, Shuhan Chen, Mengge Zhao, Nan Li, Weishuang Li, Lei Zhu. Preparation of Axially Grafted Temperature-Responsive Chiral Salen MnIII and Application in Asymmetric Epoxidation of Olefins in Water [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 244-253. |
| [9] | Honghua Zuo, Fangrui Zhong. Reactivity Modulation of Labile Quinones and Biomimetic Catalytic Transformations [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 665-678. |
| [10] | Qiang Huang, Tingting Deng, Jiayun Zhu, Jun Li, Feifei Li. Study on the Green Synthesis of β-Hydroxy-1,2,3-triazoles Catalyzed by An Amino-Functionalized Graphene-Supported Ag-Cu Composites [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 534-542. |
| [11] | Gucheng Yuan, Jiawei Liu, Shihang Yu, Chaonan Yuan, Qinghua Bian, Min Wang, Jiangchun Zhong. Asymmetric Synthesis of (3Z,6Z,9S,10R)-9,10-Epoxy-3,6-heneicosadiene, Sex Pheromone Component of Hyphantria cunea [J]. Chinese Journal of Organic Chemistry, 2021, 41(11): 4437-4443. |
| [12] | Liu Di, Zhang Chenghui, Han Nan, Du Mengmeng, Zhang Xiaolu, Zhao Pengshan, Yang Ping. Progress in Oxidative Coupling of Amines to Imines [J]. Chin. J. Org. Chem., 2018, 38(6): 1350-1363. |
| [13] | Wang Longwen, Ma Jimei, Cheng Xin, Li Zilong, Sun Linhao, Zeng Zhen, Jiang Hong. Determination of β-Glucosidase Activity Based on Enzyme-Triggered Click Chemistry [J]. Chin. J. Org. Chem., 2018, 38(10): 2775-2779. |
| [14] | Hou Shuhua, Qu Zhongguo, Zhong Keli, Bian Yanjiang, Tang Lijun. Synthesis of a Novel Benzothiazole-Rhodamine Derivative and Its Selective Detection of Fe3+, Al3+ and Cr3+ [J]. Chin. J. Org. Chem., 2016, 36(4): 768-773. |
| [15] | Han Qian, Yi Chao, Xiong Xingquan. Construction of Nitrogen-Oxygen-Heterocycles via Copper-Free Click Reactions of Nitrile Oxides [J]. Chin. J. Org. Chem., 2014, 34(6): 1092-1103. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||