Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (3): 732-741.DOI: 10.6023/cjoc202108052 Previous Articles Next Articles
REVIEWS
李可欣a, 杨庆远a,*( ), 张鹏鹏b, 张武元b,*(
), 张鹏鹏b, 张武元b,*( )
)
收稿日期:2021-08-28
修回日期:2021-09-30
发布日期:2021-10-21
通讯作者:
杨庆远, 张武元
基金资助:
Kexin Lia, Qingyuan Yanga( ), Pengpeng Zhangb, Wuyuan Zhangb(
), Pengpeng Zhangb, Wuyuan Zhangb( )
)
Received:2021-08-28
Revised:2021-09-30
Published:2021-10-21
Contact:
Qingyuan Yang, Wuyuan Zhang
Supported by:Share
Kexin Li, Qingyuan Yang, Pengpeng Zhang, Wuyuan Zhang. Research Progress of Peroxygenase-Catalyzed Reactions Driven by in-situ Generation of H2O2[J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 732-741.
| 年份 | 原位生成H2O2方式 | 催化剂/电极 | 酶 | 底物 | 产物 | eea/% | TON/TTNb | Ref. |
|---|---|---|---|---|---|---|---|---|
| 2000 | 葡萄糖氧化酶 | CPO (氯过氧化物 Chloroperoxidase) | 硫代苯甲醚 | (R)-苯甲基亚砜 | 99 | 250000 | [ | |
| 2002 | D-氨基酸 | CiP (来自灰盖鬼伞菌的过氧合酶, peroxidase from Coprinus cinereus) | (三种)芳香甲基 硫化物 | (三种)芳甲基亚砜 | 97 | >500 | [ | |
| 2007 | 甲醇氧化酶 | CiP | 硫代苯甲醚 | (R)-苯甲基亚砜 | 75左右 | >700 | [ | |
| 2014 | 甲酸脱氢酶、 NADH氧化酶 | HRP (辣根过氧合酶, peroxidase from horseradish) | 乙苯 | (R)-1-苯乙醇 | — | 6785 | [ | |
| 2015 | 酶催化 | 甲醇氧化酶、 甲醛歧化酶、 NADH氧化酶、 甲酸脱氢酶、 3-羟基苯甲酸- 6-羟化酶 | AaeUPO (真菌过加氧酶, The unspecific peroxygenase from Agrocybe aegerita) | 乙苯 | (R)-1-苯乙醇 | >99 | 294700 | [ |
| 2018 | NADH氧化酶、 YqjM | rAaeUPO (重组真菌过加氧酶, recombinant peroxygenase from Agrocybe aegerita) | 乙苯 | (R)-1-苯乙醇 | >96 | 390000 | [ | |
| 2020 | 甲酸氧化酶 | rAaeUPO | 乙苯 环己烷 顺式β-甲基苯乙烯 | (R)-1-苯乙醇 环己醇 (1R,2S)-顺-β- 甲基苯乙烯 | 97.5 | 49000 | [ | |
| 2009 | 黄素单核苷酸 | CPO | 硫代苯甲醚 | (R)-苯甲基亚砜 | <99 | 22000 | [ | |
| 2013 | 黄素单核苷酸 | CPO | 硫代苯甲醚 | (R)-苯甲基亚砜 | >96 | 12600 | [ | |
| 2017 | Au-TiO2 | rAaeUPO | 乙苯 | (R)-1-苯乙醇 | >98 | >71000 | [ | |
| 2019 | 光催化 | 石墨相氮化碳g-C3N4 | rAaeUPO | 乙苯 | (R)-1-苯乙醇 | >97.8 | 60000 | [ |
| 2019 | 酚藏红花、 亚甲基蓝、 黄素单核苷酸 | rAaeUPO | 乙苯 | (R)-1-苯乙醇 | >95 | 100000 | [ | |
| 2020 | 水溶性蒽醌 磺酸钠 | CiVCPO; rAaeUPO | 百里香酚 乙苯 环己烷 苯乙烯 4-戊烯酸 | 卤化百里香酚 (R)-1-苯乙醇 环己醇, 环己酮 1-苯基-2-溴乙醇 氧化苯乙烯 4-溴甲基环戊内酯 | 34 | 318000; 177000 | [ | |
| 2004 | 碳基电极 | CPO | 硫代苯甲醚 | (R)-苯甲基亚砜 | >98.5 | 95000 | [ | |
| 2006 | 碳基电极 | CPO | 三种底物 | 三种产物 | 93、99 | 58900 64400 7000 | [ | |
| 2007 | 碳基电极 | CPO | 硫代苯甲醚 | (R)-苯甲基亚砜 | 98.5 | 145000 | [ | |
| 2011 | 电催化 | GDE电极 | CPO | 单氯二甲醚 硫代苯甲醚 吲哚 | 二氯二甲醚 (R)-苯甲基亚砜 羟吲哚 | — | 203100 83600 39000 | [ |
| 2014 | GDE电极 | CPO | 一氯二甲酮 | 二氯二甲酮 | — | 1150000 | [ | |
| 2017 | 黄素-SWNT 电极 | AaeUPO | 乙苯 2-苯氧基丙酸 吲哚 | (R)-1-苯乙醇 | 95% | 123900±7290 5900±210 4900±340 | [ | |
| 2019 | 氧化碳纳米管 | CiVCPO | 4-戊烯酸 | 溴内酯 | — | — | [ |
| 年份 | 原位生成H2O2方式 | 催化剂/电极 | 酶 | 底物 | 产物 | eea/% | TON/TTNb | Ref. |
|---|---|---|---|---|---|---|---|---|
| 2000 | 葡萄糖氧化酶 | CPO (氯过氧化物 Chloroperoxidase) | 硫代苯甲醚 | (R)-苯甲基亚砜 | 99 | 250000 | [ | |
| 2002 | D-氨基酸 | CiP (来自灰盖鬼伞菌的过氧合酶, peroxidase from Coprinus cinereus) | (三种)芳香甲基 硫化物 | (三种)芳甲基亚砜 | 97 | >500 | [ | |
| 2007 | 甲醇氧化酶 | CiP | 硫代苯甲醚 | (R)-苯甲基亚砜 | 75左右 | >700 | [ | |
| 2014 | 甲酸脱氢酶、 NADH氧化酶 | HRP (辣根过氧合酶, peroxidase from horseradish) | 乙苯 | (R)-1-苯乙醇 | — | 6785 | [ | |
| 2015 | 酶催化 | 甲醇氧化酶、 甲醛歧化酶、 NADH氧化酶、 甲酸脱氢酶、 3-羟基苯甲酸- 6-羟化酶 | AaeUPO (真菌过加氧酶, The unspecific peroxygenase from Agrocybe aegerita) | 乙苯 | (R)-1-苯乙醇 | >99 | 294700 | [ |
| 2018 | NADH氧化酶、 YqjM | rAaeUPO (重组真菌过加氧酶, recombinant peroxygenase from Agrocybe aegerita) | 乙苯 | (R)-1-苯乙醇 | >96 | 390000 | [ | |
| 2020 | 甲酸氧化酶 | rAaeUPO | 乙苯 环己烷 顺式β-甲基苯乙烯 | (R)-1-苯乙醇 环己醇 (1R,2S)-顺-β- 甲基苯乙烯 | 97.5 | 49000 | [ | |
| 2009 | 黄素单核苷酸 | CPO | 硫代苯甲醚 | (R)-苯甲基亚砜 | <99 | 22000 | [ | |
| 2013 | 黄素单核苷酸 | CPO | 硫代苯甲醚 | (R)-苯甲基亚砜 | >96 | 12600 | [ | |
| 2017 | Au-TiO2 | rAaeUPO | 乙苯 | (R)-1-苯乙醇 | >98 | >71000 | [ | |
| 2019 | 光催化 | 石墨相氮化碳g-C3N4 | rAaeUPO | 乙苯 | (R)-1-苯乙醇 | >97.8 | 60000 | [ |
| 2019 | 酚藏红花、 亚甲基蓝、 黄素单核苷酸 | rAaeUPO | 乙苯 | (R)-1-苯乙醇 | >95 | 100000 | [ | |
| 2020 | 水溶性蒽醌 磺酸钠 | CiVCPO; rAaeUPO | 百里香酚 乙苯 环己烷 苯乙烯 4-戊烯酸 | 卤化百里香酚 (R)-1-苯乙醇 环己醇, 环己酮 1-苯基-2-溴乙醇 氧化苯乙烯 4-溴甲基环戊内酯 | 34 | 318000; 177000 | [ | |
| 2004 | 碳基电极 | CPO | 硫代苯甲醚 | (R)-苯甲基亚砜 | >98.5 | 95000 | [ | |
| 2006 | 碳基电极 | CPO | 三种底物 | 三种产物 | 93、99 | 58900 64400 7000 | [ | |
| 2007 | 碳基电极 | CPO | 硫代苯甲醚 | (R)-苯甲基亚砜 | 98.5 | 145000 | [ | |
| 2011 | 电催化 | GDE电极 | CPO | 单氯二甲醚 硫代苯甲醚 吲哚 | 二氯二甲醚 (R)-苯甲基亚砜 羟吲哚 | — | 203100 83600 39000 | [ |
| 2014 | GDE电极 | CPO | 一氯二甲酮 | 二氯二甲酮 | — | 1150000 | [ | |
| 2017 | 黄素-SWNT 电极 | AaeUPO | 乙苯 2-苯氧基丙酸 吲哚 | (R)-1-苯乙醇 | 95% | 123900±7290 5900±210 4900±340 | [ | |
| 2019 | 氧化碳纳米管 | CiVCPO | 4-戊烯酸 | 溴内酯 | — | — | [ |

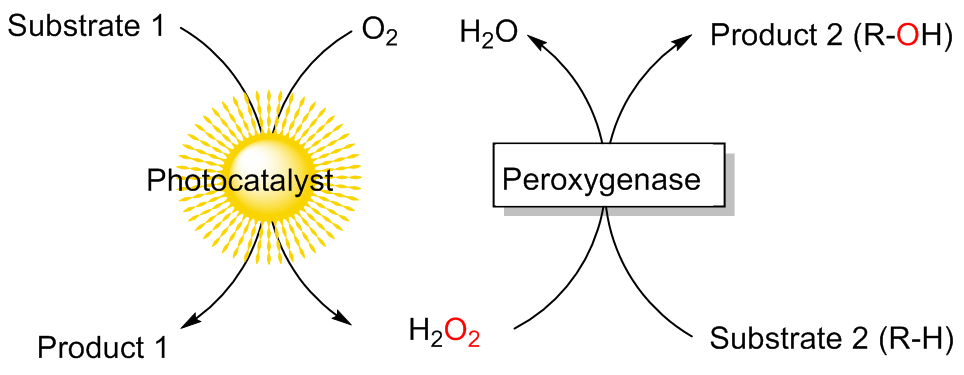
| [1] |
Dong, J.-J.; Fernández-Fueyo, E.; Hollmann, F.; Paul, C. E.; Pasic, M.; Schmidt, S.; Wang, Y.-H.; Younes, S.; Zhang, W.-Y. Angew. Chem., Int. Ed. 2018, 57, 9238.
doi: 10.1002/anie.201800343 |
| [2] |
Zeng, Z.-G.; Sang, X.-K.; Yuan, B.; Wu, M.-H.; Zhang, W.-Y. Chin. J. Org. Chem. 2021, 41, 959. (in Chinese)
doi: 10.6023/cjoc202009007 |
|
(曾志刚, 桑贤轲, 袁波, 吴鸣虎, 张武元, 有机化学, 2021, 41, 959.)
doi: 10.6023/cjoc202009007 |
|
| [3] |
Bormann, S.; Baraibar, A. G.; Ni, Y.; Holtmann, D.; Hollmann, F. Catal. Sci. Technol. 2015, 5, 2038.
doi: 10.1039/C4CY01477D |
| [4] |
Ullrich, R.; Nüske, J.; Scheibner, K.; Spantzel, J.; Hofrichter, M. Appl. Environ. Microbiol. 2004, 70, 4575.
doi: 10.1128/AEM.70.8.4575-4581.2004 |
| [5] |
Hofrichter, M.; Ullrich, R. Appl. Microbiol. Biotechnol. 2006, 71, 276.
pmid: 16628447 |
| [6] |
Zhang, W.-Y.; Hollmann, F. Synthesis of Vinyl Polymers via Enzymatic Oxidative Polymerisation, Springer Nature Singapore Pte Ltd, Singapore, 2019, p. 343.
|
| [7] |
Peter, S.; Kinne, M.; Wang, X.-S.; Ullrich, R.; Kayser, G.; Groves, J. T.; Hofrichter, M. FEBS J. 2011, 278, 3667.
doi: 10.1111/j.1742-4658.2011.08285.x |
| [8] |
Lucas, F.; Babot, E. D.; Cañellas, M.; del Río, J. C.; Kalum, L.; Ullrich, R.; Hofrichter, M.; Guallar, V.; Martínez, A. T.; Gutiérrez, A. Catal. Sci. Technol. 2016, 6, 288.
doi: 10.1039/C5CY00427F |
| [9] |
Kinne, M.; Ullrich, R.; Hammel, K. E.; Scheibner, K.; Hofrichter, M. Tetrahedron Lett. 2008, 49, 5950.
doi: 10.1016/j.tetlet.2008.07.152 |
| [10] |
de Santons, P. G.; Cañellas, M.; Tieves, F.; Younes, S. H. H.; Molina-Espeja, P.; Hofrichter, M.; Hollmann, F.; Guallar, V.; Alcalde, M. ACS Catal. 2018, 8, 4789.
doi: 10.1021/acscatal.8b01004 |
| [11] |
Molina-Espeja, P.; Cañellas, M.; Plou, F. J.; Hofrichter, M.; Lucas, F.; Guallar, V.; Alcalde, M. ChemBioChem 2016, 17, 341.
doi: 10.1002/cbic.201500493 pmid: 26677801 |
| [12] |
Thiel, D.; Doknić, D.; Deska, J. Nat. Commun. 2014, 5, 5278.
doi: 10.1038/ncomms6278 |
| [13] |
Valderrama, B.; Ayala, M.; Vazquez-Duhalt, R. Chem. Biol. 2002, 9, 555.
doi: 10.1016/S1074-5521(02)00149-7 |
| [14] |
van de Velde, F.; Lourenco, N. D.; Bakker, M.; van Rantwijk, F.; Sheldon, R. A. Biotechnol. Bioeng. 2000, 69, 286.
pmid: 10861408 |
| [15] |
Bakker, M.; van de Velde, F.; van Rantwijk, F.; Sheldon, R. A. Biotechnol. Bioeng. 2000, 70, 342.
pmid: 10992238 |
| [16] |
Okrasa, K.; Guibé-Jampel, E.; Therisod, M. J. Chem. Soc., Perkin Trans. 1 2000, 7, 1077.
|
| [17] |
Ribitsch, D.; Karl, W.; Wehrschütz-Sigl, E.; Tutz, S.; Remler, P.; Weber, H. J.; Gruber, K.; Stehr, R.; Bessler, C.; Hoven, N.; Sauter, K.; Schwab, H. Appl. Microbiol. Biotechnol. 2009, 81, 875.
doi: 10.1007/s00253-008-1661-5 pmid: 18787818 |
| [18] |
Okrasa, K.; Falcimaigne, A.; Guibé-Jampel, E.; Therisod, M. Tetrahedron: Asymmetry 2002, 13, 519.
doi: 10.1016/S0957-4166(02)00142-8 |
| [19] |
Pezzotti, F.; Okrasa, K.; Therisod, M. Tetrahedron: Asymmetry 2005, 16, 2681.
doi: 10.1016/j.tetasy.2005.07.004 |
| [20] |
Pezzotti, F.; Therisod, M. Tetrahedron: Asymmetry 2007, 18, 701.
doi: 10.1016/j.tetasy.2007.03.010 |
| [21] |
Rocha-Martin, J.; Velasco-Lozano, S.; Guisán, J. M.; López- Gallego, F. Green Chem. 2014, 16, 303.
doi: 10.1039/C3GC41456F |
| [22] |
Jung, D.; Streb, C.; Hartmann, M. Microporous Mesoporous Mater. 2008, 113, 523.
doi: 10.1016/j.micromeso.2007.12.009 |
| [23] |
Ni, Y.; Fernández-Fueyo, E.; Baraibar, A. G.; Ullrich, R.; Hofrichter, M.; Yanase, H.; Alcalde, M.; van Berkel, W. J. H.; Hollmann, F. Angew. Chem., Int. Ed. 2015, 55, 798.
doi: 10.1002/anie.201507881 |
| [24] |
Chang, A.; Scheer, M.; Grote, A.; Schomburg, I.; Schomburg, D. Nucleic Acids Res. 2009, 37, D588.
doi: 10.1093/nar/gkn820 |
| [25] |
Pesic, M; Willot, S. J. P.; Fernández-Fueyo, E.; Tieves, F.; Alcalde, M.; Hollamnn, F. Z. Naturforsch., C: J. Biosci. 2018, 74, 100.
|
| [26] |
Tieves, F.; Willot, S. J. P.; van Schie, M. M. C. H.; Rauch, M. C. R.; Younes, S. H. H.; Zhang, W.-Y.; Dong, J.-J.; de Santos, P. G.; Robbins, J. M.; Bommarius, B.; Alcalde, M.; Bommarius, A. S.; Hollmann, F. Angew. Chem., Int. Ed. 2019, 58, 7873.
doi: 10.1002/anie.v58.23 |
| [27] |
Willot, S. J. P.; Hoang, M. D.; Paul, C. E.; Alcalde, M.; Arends, I. W. C. E.; Bommarius, A. S.; Bommarius, B.; Hollmann, F. ChemCatChem 2020, 12, 2713.
doi: 10.1002/cctc.v12.10 |
| [28] |
Li, Y.-Y.; Yuan, B.; Sun, Z.-T.; Zhang, W.-Y. Green Synth. Catal. 2021, 2, 267.
|
| [29] |
Perez, D. I.; Grau, M. M.; Arends, I. W. C. E.; Hollmann, F. Chem. Commun. 2009, 41, 6848.
|
| [30] |
Churakova, E.; Kluge, M.; Ullrich, R.; Arends, I.; Hofrichter, M.; Hollmann, F. Angew. Chem., Int. Ed. 2011, 50, 10716.
doi: 10.1002/anie.201105308 |
| [31] |
Churakova, E.; Arends, I. W. C. E.; Hollmann, F. ChemCatChem 2013, 5, 565.
doi: 10.1002/cctc.201200490 |
| [32] |
Zhang, W.-Y.; Burek, B. O.; Fernández-Fueyo, E.; Alcalde, M.; Bloh, J. Z.; Hollmann, F. Angew. Chem. 2017, 56, 15451.
doi: 10.1002/anie.201708668 |
| [33] |
Zhang, W.-Y.; Fernández-Fueyo, E.; Ni, Y.; van Schie, M.; Gacs, J.; Renirie, R.; Wever, R.; Mutti, F. G.; Rother, D. R.; Alcalde, M.; Hollmann, F. Nat. Catal. 2018, 1, 55.
doi: 10.1038/s41929-017-0001-5 |
| [34] |
Teranishi, M.; Hoshino, R.; Naya, S.; Tada, H. Angew. Chem. 2016, 128, 12965.
doi: 10.1002/ange.201606734 |
| [35] |
van Schie, M. M. C. H.; Zhang, W.-Y.; Tieves, F.; Choi, D. S.; Park, C. B.; Burek, B. O.; Bloh, J. Z.; Arends, I. W. C. E.; Paul, C. E.; Alcalde, M.; Hollmann, F. ACS Catal. 2019, 9, 7409.
doi: 10.1021/acscatal.9b01341 |
| [36] |
Willot, S. J. P.; Fernández-Fueyo, E.; Tieves, F.; Pesic, M.; Alcalde, M.; Arends, I. W. C. E.; Park, C. B.; Hollmann, F. ACS Catal. 2019, 9, 890.
doi: 10.1021/acscatal.8b03752 |
| [37] |
Yuan, B.; Mahor, D.; Fei, Q.; Wever, R.; Alcalde, M.; Zhang, W.-Y.; Hollmann, F. ACS Catal. 2020, 10, 8277.
doi: 10.1021/acscatal.0c01958 pmid: 32802571 |
| [38] |
Zhang, W.-Y.; Liu, H.-H.; van Schie, M. M. C. H.; Hagedoorn, P. L.; Alcalde, M.; Denkova, A. G.; Djanashvili, K.; Hollmann, F. ACS Catal. 2020, 10, 14195.
doi: 10.1021/acscatal.0c03059 |
| [39] |
Lvtz, S.; Steckhan, E.; Liese, A. Electrochem. Commun. 2004, 6, 583.
doi: 10.1016/j.elecom.2004.04.009 |
| [40] |
Kohlmann, C.; Lvtz, S. Eng. Life Sci. 2006, 6, 170.
doi: 10.1002/(ISSN)1618-2863 |
| [41] |
Lvtz, S.; Vuorilehto, K.; Liese, A. Biotechnol. Bioeng. 2007, 98, 525.
doi: 10.1002/bit.v98:3 |
| [42] |
Horst, A. E. M.; Mangold, K. M.; Holtmann, D. Biotechnol. Bioeng. 2016, 113, 260.
doi: 10.1002/bit.25698 |
| [43] |
Krieg, T.; Hvttmann, S.; Mangold, K. M.; Schrader, J.; Holtmann, D. Green Chem. 2011, 13, 2686.
doi: 10.1039/c1gc15391a |
| [44] |
Holtmann, D.; Krieg, T.; Getrey, L.; Schrader, J. Catal. Commun. 2014, 51, 82.
doi: 10.1016/j.catcom.2014.03.033 |
| [45] |
Horst, A. E. W.; Bormann, S.; Meyer, J.; Steinhagen, M.; Ludwig, R.; Drews, A.; Ansorge-Schumacher, M.; Holtmann, D. J. Mol. Catal. B: Enzym. 2016, 133, S137.
doi: 10.1016/j.molcatb.2016.12.008 |
| [46] |
Choi, D. S.; Ni, Y.; Fernández-Fueyo, E.; Lee, M.; Hollmann, F.; Park, C. B. ACS Catal. 2017, 7, 1563.
doi: 10.1021/acscatal.6b03453 |
| [47] |
Kim, H. W.; Ross, M. B.; Kornienko, N.; Zhang, L.; Guo, J.-H.; Yang, P.-D.; McCloskey, B. D. Nat. Catal. 2018, 1, 282.
doi: 10.1038/s41929-018-0044-2 |
| [48] |
Bormann, S.; van Schie, M. M. C. H.; De Almeida, T. P.; Zhang, W.-Y.; Stöckl, M.; Ulber, R.; Hollmann, F.; Holtmann, D. ChemSusChem 2019, 12, 4759.
doi: 10.1002/cssc.v12.21 |
| [49] |
Karmee, S. K.; Roosen, C.; Kohlmann, C.; Lvtz, S.; Greiner, L.; Leitner, W. Green Chem. 2009, 11, 1052.
doi: 10.1039/b820606f |
| [50] |
Edwards, J. K.; Freakley, S. J.; Carley, A. F.; Kiely, C. J.; Hutchings, G. J. Acc. Chem. Res. 2014, 47, 845.
doi: 10.1021/ar400177c |
| [51] |
Freakley, S. J.; Kochius, S.; van Marwijk, J.; Fenner, C.; Lewis, R. J.; Baldenius, K.; Marais, S. S.; Opperman, D. J.; Harrison, S. T. L.; Alcalde, M.; Smit, M. S.; Hutchings, G. J. Nat. Commun. 2019, 10, 1.
doi: 10.1038/s41467-018-07882-8 |
| [52] |
Yoon, J.; Kim, J.; Tieves, F.; Zhang, W.-Y.; Alcalde, M.; Hollmann, F.; Park, C. B. ACS Catal. 2020, 10, 5236.
doi: 10.1021/acscatal.0c00188 |
| [1] | Luyao Li, Zhongwen He, Zhenguo Zhang, Zhenhua Jia, Teck-Peng Loh. Application of Triaryl Carbenium in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 421-437. |
| [2] | Qianfan Zhao, Yongzheng Chen, Shiming Zhang. Application and Mechanism Study of Carbon-Based Metal-Free Catalysts in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 137-147. |
| [3] | Ran Zhou, Chunmei Yuan, Tao Zhang, Piao Mao, Yi Liu, Kaini Meng, Hui Xin, Wei Xue. Design, Synthesis and Bioactivity of Chalcone Derivative Containing Quinazolinone [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3196-3209. |
| [4] | Guangli Xu, Jing Xu, Haidong Xu, Xiang Cui, Xingzhong Shu. Research Progress of Transition Metal Catalyzed Synthesis of 1,3- Conjugated Diene Compounds from Alkenes and Alkynes [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1899-1933. |
| [5] | Linsheng Bai, Peng Hong, Anguo Ying. Research Progress of Functional Polyacrylonitrile Fiber in Promoting Organic Reaction [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1241-1270. |
| [6] | Baichuan Mo, Chunxia Chen, Jinsong Peng. Research Progress in Application of Lignin and Its Derivatives Supported Metal Catalysts in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1215-1240. |
| [7] | Qian Dou, Taimin Wang, Lijing Fang, Hongbin Zhai, Bin Cheng. Recent Development of Photoinduced Iron-Catalysis in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1386-1415. |
| [8] | Shiquan Gao, Chuangjun Liu, Junfeng Yang, Junliang Zhang. Cobalt-Catalyzed Electrochemical Reductive Coupling of Alkynes and Alkenes [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1559-1565. |
| [9] | Biao Ma, Miaomiao Zhang, Zhanyu Li, Jinsong Peng, Chunxia Chen. Recent Advance of Transition Metal-Free Catalyzed Suzuki-Type Cross Coupling Reaction [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 455-470. |
| [10] | Silin Chen, Yunhui Yang, Chao Chen, Congyang Wang. Advances in Transition-Metal-Catalyzed Keto Carbonyl-Directed C—H Bond Functionalization Reactions [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 1-16. |
| [11] | Runye Gao, Lingling Zuo, Fang Wang, Chuanying Li, Huajiang Jiang, Pinhua Li, Lei Wang. Recent Advances in Controllable Organic Reactions Induced by Visible Light without External Photocatalyst [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 1883-1903. |
| [12] | Weichun Huang, Xinyu Ding, You Zi. Research Progress of Vinyl/Aryl Phosphonium Salts in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 471-486. |
| [13] | Siyu Mu, Hongxia Li, Zhilin Wu, Junmei Peng, Jinyang Chen, Weimin He. Electrocatalytic Three-Component Synthesis of 4-Bromopyrazoles from Acetylacetone, Hydrazine and Diethyl Bromomalonate [J]. Chinese Journal of Organic Chemistry, 2022, 42(12): 4292-4299. |
| [14] | Shaohui Yang, Jingcheng Song, Daoqing Dong, Hao Yang, Mengyu Zhou, Huishu Zhang, Zuli Wang. Progress of N-Amino Pyridinium Salts as Nitrogen Radical Precursors in Visible Light Induced C—N Bond Formation Reactions [J]. Chinese Journal of Organic Chemistry, 2022, 42(12): 4099-4110. |
| [15] | Rongnan Yi, Dongxian Liu, Qilin Wu, Mingming Zhao, Yong Wang, Zheng Wang. Electrochemical Oxidated-Iodide Promoted α-H Aryl(alkyl)selenation of Acetone for the Preparation of α-Aryl(alkyl)selenoacetones [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3726-3732. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||