Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (2): 543-556.DOI: 10.6023/cjoc202107002 Previous Articles Next Articles
ARTICLES
曾艳a,b, 聂礼飞a, 牛超a, 阿依提拉•麦麦提江a,b, Khurshed Bozorova, 赵江瑜a, 阿吉艾克拜尔•艾萨a,b,*( )
)
收稿日期:2021-07-02
修回日期:2021-09-06
发布日期:2022-02-24
通讯作者:
阿吉艾克拜尔•艾萨
基金资助:
Yan Zenga,b, Lifei Niea, Chao Niua, Aytilla Mamatjana,b, Khurshed Bozorova, Jiangyu Zhaoa, Haji Akber Aisaa,b( )
)
Received:2021-07-02
Revised:2021-09-06
Published:2022-02-24
Contact:
Haji Akber Aisa
Supported by:Share
Yan Zeng, Lifei Nie, Chao Niu, Aytilla Mamatjan, Khurshed Bozorov, Jiangyu Zhao, Haji Akber Aisa. Synthesis and Biological Activities of Dihydrooxazolo[5,4-d]-pyrrolo[1,2-a]pyrimidinones[J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 543-556.

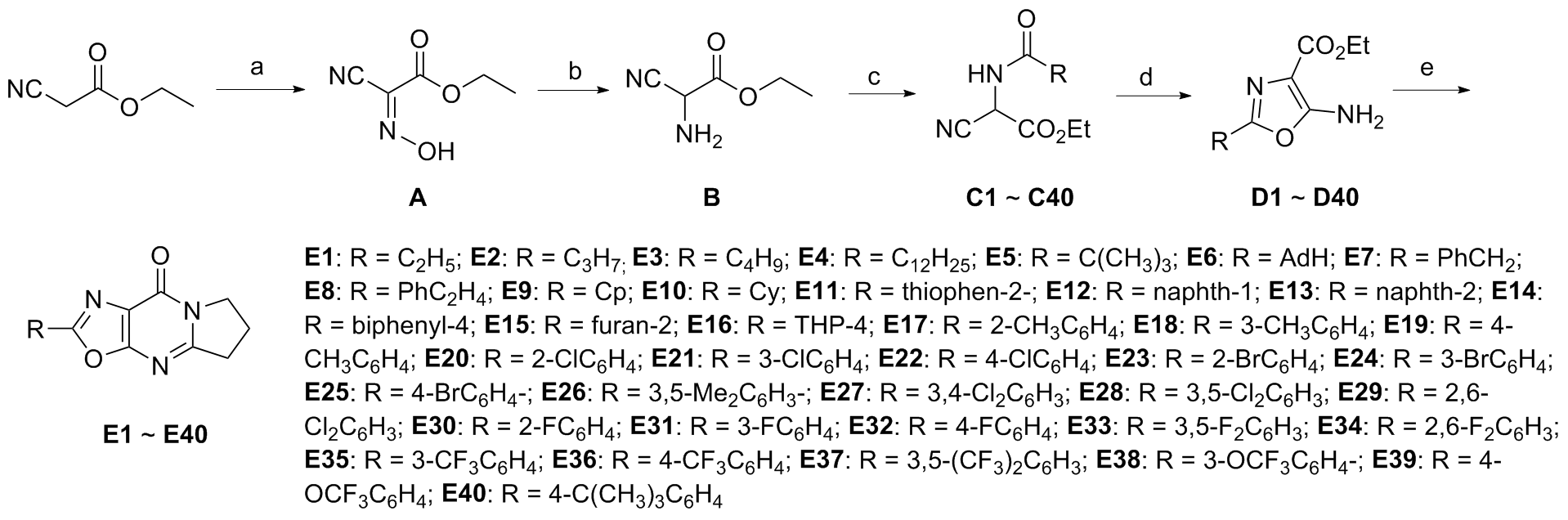
| Entry | POCl3/equiv. | Solvent (dry) | Temp./℃ | Yield/% |
|---|---|---|---|---|
| 1 | 2 | DCM | Reflux | — |
| 2 | 2 | DCE | Reflux | 54 |
| 3 | 2 | THF | Reflux | — |
| 4 | 2 | CH3CN | Reflux | — |
| 5 | 2 | CH3OH | Reflux | — |
| 6 | 2 | PhCH3 | Reflux | 51 |
| 7 | 2 | 1,4-Dioxane | Reflux | 67 |
| 8 | 1 | 1,4-Dioxane | Reflux | 48 |
| 9 | 3 | 1,4-Dioxane | Reflux | 73 |
| 10 | 5 | 1,4-Dioxane | Reflux | 71 |
| 11 | 3 | 1,4-Dioxane | 20 | 34 |
| 12 | 3 | 1,4-Dioxane | 40 | 38 |
| 13 | 3 | 1,4-Dioxane | 60 | 56 |
| 14 | 3 | 1,4-Dioxane | 80 | 82 |
| 15 | 3 | 1,4-Dioxane | 100 | 79 |
| Entry | POCl3/equiv. | Solvent (dry) | Temp./℃ | Yield/% |
|---|---|---|---|---|
| 1 | 2 | DCM | Reflux | — |
| 2 | 2 | DCE | Reflux | 54 |
| 3 | 2 | THF | Reflux | — |
| 4 | 2 | CH3CN | Reflux | — |
| 5 | 2 | CH3OH | Reflux | — |
| 6 | 2 | PhCH3 | Reflux | 51 |
| 7 | 2 | 1,4-Dioxane | Reflux | 67 |
| 8 | 1 | 1,4-Dioxane | Reflux | 48 |
| 9 | 3 | 1,4-Dioxane | Reflux | 73 |
| 10 | 5 | 1,4-Dioxane | Reflux | 71 |
| 11 | 3 | 1,4-Dioxane | 20 | 34 |
| 12 | 3 | 1,4-Dioxane | 40 | 38 |
| 13 | 3 | 1,4-Dioxane | 60 | 56 |
| 14 | 3 | 1,4-Dioxane | 80 | 82 |
| 15 | 3 | 1,4-Dioxane | 100 | 79 |
| Compd. | R | IC50/(μmol•L–1) | Diameter of antibacterial cycle/mm | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hela | MCF-7 | A549 | C.a.a | E.c.b | S.a.c | |||||||||||
| E1 | C2H5 | >50 | >50 | >50 | — | — | — | |||||||||
| E2 | C3H7 | >50 | >50 | >50 | — | — | — | |||||||||
| E3 | C4H9 | >50 | >50 | >50 | — | — | — | |||||||||
| E4 | C12H25 | 43.61±7.34 | >50 | >50 | — | — | — | |||||||||
| E5 | C(CH3)3 | >50 | >50 | >50 | — | — | — | |||||||||
| E6 | AdH | >50 | >50 | >50 | 9 | 10.5 | 10 | |||||||||
| E7 | CH2Ph | >50 | >50 | >50 | 9 | — | — | |||||||||
| E8 | C2H4Ph | 31.44±0.1 | >50 | >50 | 10 | — | — | |||||||||
| E9 | Cp | 30.94±0.7 | >50 | >50 | — | — | — | |||||||||
| E10 | Cy | >50 | >50 | >50 | — | — | — | |||||||||
| E11 | Thiophen-2 | 48.7±0.1 | >50 | >50 | — | — | — | |||||||||
| E12 | Naphth-1 | 4.59±0.10 | >50 | 18.66±0.1 | — | — | — | |||||||||
| E13 | Naphth-2 | 34.82±0.2 | >50 | >50 | — | — | — | |||||||||
| E14 | Biphenyl-4 | >50 | >50 | 18.40±0.1 | — | — | — | |||||||||
| E15 | Furan-2 | >50 | >50 | >50 | — | — | — | |||||||||
| E16 | THP-4 | >50 | >50 | >50 | — | — | — | |||||||||
| E17 | 2-CH3C6H4 | >50 | 43.42±4.99 | >50 | — | — | — | |||||||||
| E18 | 3-CH3C6H4 | >50 | >50 | >50 | 8.5 | — | — | |||||||||
| E19 | 4-CH3C6H4 | 13.46±0.41 | 19.34±3.84 | >50 | — | — | — | |||||||||
| E20 | 2-ClC6H4 | >50 | >50 | >50 | 13 | — | — | |||||||||
| E21 | 3-ClC6H4 | >50 | >50 | >50 | 8.5 | — | — | |||||||||
| Compd. | R | IC50/(μmol•L–1) | Diameter of antibacterial cycle/mm | |||||||||||||
| Hela | MCF-7 | A549 | C.a.a | E.c.b | S.a.c | |||||||||||
| E22 | 4-ClC6H4 | >50 | >50 | >50 | 8.5 | — | — | |||||||||
| E23 | 2-BrC6H4 | >50 | >50 | >50 | — | — | — | |||||||||
| E24 | 3-BrC6H4 | >50 | >50 | >50 | — | — | — | |||||||||
| E25 | 4-BrC6H4 | >50 | >50 | >50 | — | — | 9 | |||||||||
| E26 | 3,5-Me2C6H3 | >50 | >50 | >50 | — | 11 | — | |||||||||
| E27 | 3,4-Cl2C6H3 | >50 | >50 | >50 | 9 | 14 | — | |||||||||
| E28 | 3,5-Cl2C6H3 | >50 | >50 | >50 | 10 | 10 | 9.5 | |||||||||
| E29 | 2,6-Cl2C6H3 | >50 | >50 | >50 | — | — | — | |||||||||
| E30 | 2-FC6H4 | >50 | >50 | >50 | — | 11 | — | |||||||||
| E31 | 3-FC6H4 | >50 | >50 | >50 | — | — | — | |||||||||
| E32 | 4-F C6H4 | >50 | >50 | >50 | — | — | — | |||||||||
| E33 | 3,5-F2C6H3 | >50 | >50 | >50 | — | — | 8 | |||||||||
| E34 | 2,6-F2C6H3 | >50 | >50 | >50 | — | 10 | — | |||||||||
| E35 | 3-CF3C6H4 | 36.86±4.48 | >50 | >50 | — | — | — | |||||||||
| E36 | 4-CF3C6H4 | 42.17±0.85 | >50 | >50 | — | — | — | |||||||||
| E37 | 3,5-(CF3)2C6H3 | >50 | >50 | >50 | — | 10 | 9.5 | |||||||||
| E38 | 3-CF3OC6H4 | >50 | >50 | >50 | — | 10 | — | |||||||||
| E39 | 4-CF3OC6H4 | >50 | 6.89±1.26 | >50 | — | 10 | — | |||||||||
| E40 | 4-(CH3)3CC6H4 | >50 | 30.28±9.66 | >50 | — | 10 | — | |||||||||
| DMSOd | NDe | ND | ND | |||||||||||||
| 调零f | ND | ND | ND | |||||||||||||
| DOX | 0.23±0.15 | 0.15±0.01 | 0.34±0.03 | |||||||||||||
| CPT | 1.32±0.08 | 3.47±0.11 | 0.81±0.14 | |||||||||||||
| 氨苄青霉素 | 11.5g | |||||||||||||||
| 氨苄青霉素 | 10h | |||||||||||||||
| 两性霉素B | 14i | |||||||||||||||
| Compd. | R | IC50/(μmol•L–1) | Diameter of antibacterial cycle/mm | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hela | MCF-7 | A549 | C.a.a | E.c.b | S.a.c | |||||||||||
| E1 | C2H5 | >50 | >50 | >50 | — | — | — | |||||||||
| E2 | C3H7 | >50 | >50 | >50 | — | — | — | |||||||||
| E3 | C4H9 | >50 | >50 | >50 | — | — | — | |||||||||
| E4 | C12H25 | 43.61±7.34 | >50 | >50 | — | — | — | |||||||||
| E5 | C(CH3)3 | >50 | >50 | >50 | — | — | — | |||||||||
| E6 | AdH | >50 | >50 | >50 | 9 | 10.5 | 10 | |||||||||
| E7 | CH2Ph | >50 | >50 | >50 | 9 | — | — | |||||||||
| E8 | C2H4Ph | 31.44±0.1 | >50 | >50 | 10 | — | — | |||||||||
| E9 | Cp | 30.94±0.7 | >50 | >50 | — | — | — | |||||||||
| E10 | Cy | >50 | >50 | >50 | — | — | — | |||||||||
| E11 | Thiophen-2 | 48.7±0.1 | >50 | >50 | — | — | — | |||||||||
| E12 | Naphth-1 | 4.59±0.10 | >50 | 18.66±0.1 | — | — | — | |||||||||
| E13 | Naphth-2 | 34.82±0.2 | >50 | >50 | — | — | — | |||||||||
| E14 | Biphenyl-4 | >50 | >50 | 18.40±0.1 | — | — | — | |||||||||
| E15 | Furan-2 | >50 | >50 | >50 | — | — | — | |||||||||
| E16 | THP-4 | >50 | >50 | >50 | — | — | — | |||||||||
| E17 | 2-CH3C6H4 | >50 | 43.42±4.99 | >50 | — | — | — | |||||||||
| E18 | 3-CH3C6H4 | >50 | >50 | >50 | 8.5 | — | — | |||||||||
| E19 | 4-CH3C6H4 | 13.46±0.41 | 19.34±3.84 | >50 | — | — | — | |||||||||
| E20 | 2-ClC6H4 | >50 | >50 | >50 | 13 | — | — | |||||||||
| E21 | 3-ClC6H4 | >50 | >50 | >50 | 8.5 | — | — | |||||||||
| Compd. | R | IC50/(μmol•L–1) | Diameter of antibacterial cycle/mm | |||||||||||||
| Hela | MCF-7 | A549 | C.a.a | E.c.b | S.a.c | |||||||||||
| E22 | 4-ClC6H4 | >50 | >50 | >50 | 8.5 | — | — | |||||||||
| E23 | 2-BrC6H4 | >50 | >50 | >50 | — | — | — | |||||||||
| E24 | 3-BrC6H4 | >50 | >50 | >50 | — | — | — | |||||||||
| E25 | 4-BrC6H4 | >50 | >50 | >50 | — | — | 9 | |||||||||
| E26 | 3,5-Me2C6H3 | >50 | >50 | >50 | — | 11 | — | |||||||||
| E27 | 3,4-Cl2C6H3 | >50 | >50 | >50 | 9 | 14 | — | |||||||||
| E28 | 3,5-Cl2C6H3 | >50 | >50 | >50 | 10 | 10 | 9.5 | |||||||||
| E29 | 2,6-Cl2C6H3 | >50 | >50 | >50 | — | — | — | |||||||||
| E30 | 2-FC6H4 | >50 | >50 | >50 | — | 11 | — | |||||||||
| E31 | 3-FC6H4 | >50 | >50 | >50 | — | — | — | |||||||||
| E32 | 4-F C6H4 | >50 | >50 | >50 | — | — | — | |||||||||
| E33 | 3,5-F2C6H3 | >50 | >50 | >50 | — | — | 8 | |||||||||
| E34 | 2,6-F2C6H3 | >50 | >50 | >50 | — | 10 | — | |||||||||
| E35 | 3-CF3C6H4 | 36.86±4.48 | >50 | >50 | — | — | — | |||||||||
| E36 | 4-CF3C6H4 | 42.17±0.85 | >50 | >50 | — | — | — | |||||||||
| E37 | 3,5-(CF3)2C6H3 | >50 | >50 | >50 | — | 10 | 9.5 | |||||||||
| E38 | 3-CF3OC6H4 | >50 | >50 | >50 | — | 10 | — | |||||||||
| E39 | 4-CF3OC6H4 | >50 | 6.89±1.26 | >50 | — | 10 | — | |||||||||
| E40 | 4-(CH3)3CC6H4 | >50 | 30.28±9.66 | >50 | — | 10 | — | |||||||||
| DMSOd | NDe | ND | ND | |||||||||||||
| 调零f | ND | ND | ND | |||||||||||||
| DOX | 0.23±0.15 | 0.15±0.01 | 0.34±0.03 | |||||||||||||
| CPT | 1.32±0.08 | 3.47±0.11 | 0.81±0.14 | |||||||||||||
| 氨苄青霉素 | 11.5g | |||||||||||||||
| 氨苄青霉素 | 10h | |||||||||||||||
| 两性霉素B | 14i | |||||||||||||||
| [1] |
Alshamma, A.; Drake, S.; Flynn, D. L.; Mitscher, L. A.; Park, Y. H.; Rao, G. S. R.; Simpson, A.; Swayze, J. K.; Veysoglu, T.; Wu, S. T. - S. J. Nat. Prod. 1981, 44, 745.
pmid: 7334386 |
| [2] |
Mohr, N.; Budzikiewicz, H.; Korth, H.; Pulverer, und. G. Liebigs Ann. Chem. 1981, 1515.
|
| [3] |
Okmanov, R. Y.; Tashkhodzhaev, B.; Khakimova, Z. M.; Tulyaganov, T. S.; Shakhidoyatov, K. M. Chem. Nat. Compd. 2010, 46, 59.
doi: 10.1007/s10600-010-9524-6 |
| [4] |
Wu, X.-Y.; Qin, G.-W.; Cheung, K. K.; Cheng, K. F. Tetrahedron 1997, 53, 13323.
doi: 10.1016/S0040-4020(97)00846-6 |
| [5] |
Molina, P.; Tárraga, A.; Gonzalez-Tejero, A.; Rioja, I.; Ubeda, A.; Terencio, M. C.; Alcaraz, M. J. J. Nat. Prod. 2001, 64, 1297.
pmid: 11678654 |
| [6] |
Wang, C.-H.; Zeng, H.; Wang, Y.-H.; Li, C.; Cheng, J.; Ye, Z.-J.; He, X.-J. J. Asian Nat. Prod. Res. 2015, 17, 595.
doi: 10.1080/10286020.2015.1042373 |
| [7] |
Potewar, T. M.; Ingale, S. A.; Srinivasan, K. V. ARKIVOC 2008, 100.
|
| [8] |
Nepali, K.; Sharma, S.; Ojha, R.; Dhar, K. L. Med. Chem. Res. 2013, 22, 1.
doi: 10.1007/s00044-012-0002-5 |
| [9] |
Zhang, H.-Z.; Zhao, Z.-L.; Zhou, C.-H. Eur. J. Med. Chem. 2018, 144, 444.
doi: 10.1016/j.ejmech.2017.12.044 |
| [10] |
Zhang, H.-Z.; Gan, L.-L.; Wang, H.; Zhou, C.-H. Mini-Rev. Med. Chem. 2017, 17, 122.
doi: 10.2174/1389557516666160630120725 |
| [11] |
Zhang, L.; Peng, X.-M.; Damu, G. L. V.; Geng, R.-X.; Zhou, C.-H. Med. Res. Rev. 2014, 34, 340.
doi: 10.1002/med.21290 pmid: 23740514 |
| [12] |
Zhou, C.-H.; Wang, Y. Curr. Med. Chem. 2012, 19, 239.
pmid: 22320301 |
| [13] |
Seenaiah, D.; Reddy, P. R.; Reddy, G. M.; Padmaja, A.; Padmavathi, V.; Krishna, N. S. Eur. J. Med. Chem. 2014, 77, 1.
doi: 10.1016/j.ejmech.2014.02.050 pmid: 24607584 |
| [14] |
Tropcheva, R.; Lesev, N.; Danova, S.; Stoitsova, S.; Kaloyanova, S. J. Photochem. Photobiol.,B 2015, 143, 120.
doi: 10.1016/j.jphotobiol.2015.01.002 |
| [15] |
Zhang, Y.; Bao, J.; Deng, X.-X.; He, W.; Fan, J.-J.; Jiang, F.-Q.; Fu, L. Bioorg. Med. Chem. Lett. 2016, 26, 4081.
doi: 10.1016/j.bmcl.2016.06.074 pmid: 27400889 |
| [16] |
Li, Z.; Gever, J. R.; Rao, S.; Widjaja, K.; Prusiner, S. B.; Silber, B. M. ACS Med. Chem. Lett. 2013, 4, 397.
doi: 10.1021/ml300472n |
| [17] |
Bernard, A. M.; Cabiddu, M. G.; De Montis, S.; Mura, R.; Pompei, R. Biorg. Med. Chem. 2014, 22, 4061.
doi: 10.1016/j.bmc.2014.05.066 |
| [18] |
Henderson, J. A.; Bilimoria, D.; Bubenik, M.; Cadilhac, C.; Cottrell, K. M.; Denis, F.; Dietrich, E.; Ewing, N.; Falardeau, G.; Giroux, S.; L'Heureux, L.; Liu, B.; Mani, N.; Morris, M.; Nicolas, O.; Pereira, O. Z.; Poisson, C.; Reddy, T. J.; Selliah, S.; Shawgo, R. S.; Vaillancourt, L.; Wang, J.; Xu, J.; Chauret, N.; Berlioz-Seux, F.; Chan, L. C.; Das, S. K.; Grillot, A.-L.; Bennani, Y. L.; Maxwell, J. P. Biorg. Med. Chem. Lett. 2015, 25, 948.
doi: 10.1016/j.bmcl.2014.12.042 |
| [19] |
Lu, X.-Y.; Hu, X.-L.; Liu, Z.-Y.; Zhang, T.-Y.; Wang, R.-B.; Wan, B.-J.; Franzblau, S. G.; You, Q.-D. MedChemComm 2017, 8, 1303.
doi: 10.1039/C7MD00146K |
| [20] |
Costales, A.; Mathur, M.; Ramurthy, S.; Lan, J.; Subramanian, S.; Jain, R.; Atallah, G.; Setti, L.; Lindvall, M.; Appleton, B. A.; Ornelas, E.; Feucht, P.; Warne, B.; Doyle, L.; Basham, S. E.; Aronchik, I.; Jefferson, A. B.; Shafer, C. M. Bioorg. Med. Chem. Lett. 2014, 24, 1592.
doi: 10.1016/j.bmcl.2014.01.058 pmid: 24534486 |
| [21] |
An, Y.; Lee, E.; Yu, Y.; Yun, J.; Lee, M. Y.; Kang, J. S.; Kim, W.-Y.; Jeon, R. Bioorg. Med. Chem. Lett. 2016, 26, 3067.
doi: 10.1016/j.bmcl.2016.05.017 |
| [22] |
Zhang, J.; Yao, D.-H.; Jiang, Y.-N.; Huang, J.; Yang, S.-L.; Wang, J.-H. Bioorg. Chem. 2017, 72, 168.
doi: S0045-2068(17)30063-9 pmid: 28460359 |
| [23] |
Seth, K.; Garg, S. K.; Kumar, R.; Purohit, P.; Meena, V. S.; Goyal, R.; Banerjee, U. C.; Chakraborti, A. K. ACS Med. Chem. Lett. 2014, 5, 512.
doi: 10.1021/ml400500e |
| [24] |
Salomé, C.; Ribeiro, N.; Chavagnan, T.; Thuaud, F.; Serova, M.; de Gramont, A.; Faivre, S.; Raymond, E.; Désaubry, L. Eur. J. Med. Chem. 2014, 81, 181.
doi: 10.1016/j.ejmech.2014.05.014 |
| [25] |
Lu, X.-Y.; Liu, X.-B.; Wan, B.-J.; Franzblau, S. G.; Chen, L.-L.; Zhou, C.-L.; You, Q.-D. Eur. J. Med. Chem. 2012, 49, 164.
doi: 10.1016/j.ejmech.2012.01.007 |
| [1] | Fakai Zou, Nengzhong Wang, Hui Yao, Hui Wang, Mingguo Liu, Nianyu Huang. Regio- and Stereo-selective Synthesis of 1β-/3R-Aryl Thiosugar [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 593-604. |
| [2] | Luyao Li, Zhongwen He, Zhenguo Zhang, Zhenhua Jia, Teck-Peng Loh. Application of Triaryl Carbenium in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 421-437. |
| [3] | Qinggang Mei, Qinghan Li. Recent Progress of Visible Light-Induced the Synthesis of C(3) (Hetero)arylthio Indole Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 398-408. |
| [4] | Penghui Li, Qingyang Xie, Fuxian Wan, Yuanhong Zhang, Lin Jiang. Synthesis and Fungicidal Activity of Novel Substituted Pyrimidine-5-carboxamides Bearing Cyclopropyl Moiety [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 650-656. |
| [5] | Weiqing Yang, Yanbing Ge, Yuanyuan Chen, Ping Liu, Haiyan Fu, Menglin Ma. Design and Synthesis of Fluorescent 1,8-Napthalimide Derivatives and Their Identification of Cysteine [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 180-194. |
| [6] | Qianfan Zhao, Yongzheng Chen, Shiming Zhang. Application and Mechanism Study of Carbon-Based Metal-Free Catalysts in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 137-147. |
| [7] | Shan Chen, Zhilin Chen, Qiong Hu, Yanshuang Meng, Yue Huang, Pingfang Tao, Liru Lu, Guobao Huang. Recognition of Bis-thiourea Tweezers to Neutral Molecules in Non-Polar Solvent [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 277-281. |
| [8] | Huakun Wang, Xiaolong Ren, Yining Xuan. Study of the Halide Salt Catalyzed [3+2] Cycloaddition of α,β-Epoxy Carboxylate with Isocyanate [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 251-258. |
| [9] | Yukun Jin, Baoyi Ren, Fushun Liang. Visible Light-Mediated Selective C—F Bond Cleavage of Trifluoromethyl Groups and Its Application in Synthesizing gem-Difluoro-Containing Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 85-110. |
| [10] | Cuiyun Ma, Hailan Luo, Fuhua Zhang, Dan Guo, Shuxing Chen, Fei Wang. Green Biosynthesis, Photophysical Properties and Application of 3-Pyrrolyl BODIPY [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 216-223. |
| [11] | Bozhen Wang, Jie Zhang, Chunhui Nian, Mingming Jin, Miaomiao Kong, Wulan Li, Wenfei He, Jianzhang Wu. Synthesis and Antitumor Activity of 3,4-Dichlorophenyl Amides [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 232-241. |
| [12] | Shihang Yu, Jiawei Liu, Biyu An, Qinghua Bian, Min Wang, Jiangchun Zhong. Asymmetric Synthesis of the Contact Sex Pheromone of Neoclytus acuminatus acuminatus (Fabricius) [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 301-308. |
| [13] | Huanqing Li, Zhaohua Chen, Zujia Chen, Qiwen Qiu, Youcai Zhang, Sihong Chen, Zhaoyang Wang. Research Progress in Mercury Ion Fluorescence Probes Based on Organic Small Molecules [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3067-3077. |
| [14] | Ran Zhou, Chunmei Yuan, Tao Zhang, Piao Mao, Yi Liu, Kaini Meng, Hui Xin, Wei Xue. Design, Synthesis and Bioactivity of Chalcone Derivative Containing Quinazolinone [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3196-3209. |
| [15] | Ruixia Cao, Yuping Jia. Synthesis and Biological Activity of Novel Pyrrolo[2,3-d]pyrimidine Derivatives Containing Coumarin [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3304-3311. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||