Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (7): 1904-1924.DOI: 10.6023/cjoc202202005 Previous Articles Next Articles
REVIEWS
收稿日期:2022-02-06
修回日期:2022-03-23
发布日期:2022-08-09
通讯作者:
苗志伟
基金资助:
Yuanhao Maoa, Yanfeng Gaoa, Zhiwei Miaoa,b( )
)
Received:2022-02-06
Revised:2022-03-23
Published:2022-08-09
Contact:
Zhiwei Miao
Supported by:Share
Yuanhao Mao, Yanfeng Gao, Zhiwei Miao. Research Progress on the Asymmetric Cyclization Synthesis of Seven-Membered Rings via Transition Metal Catalysis[J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 1904-1924.
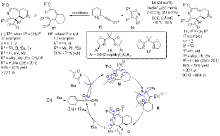
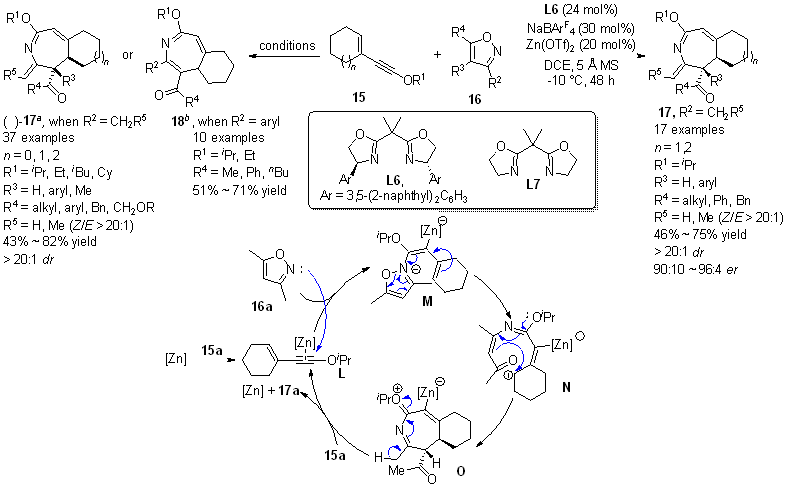
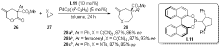
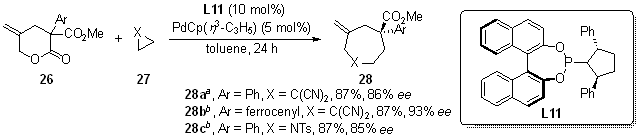

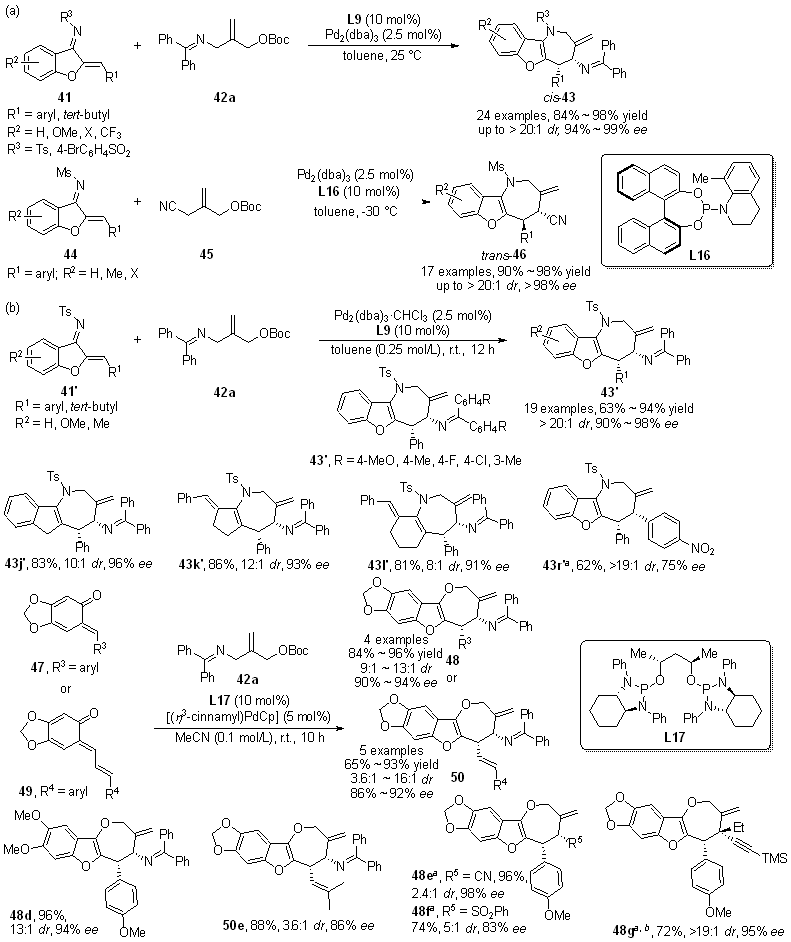
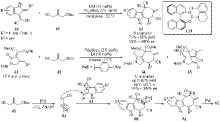
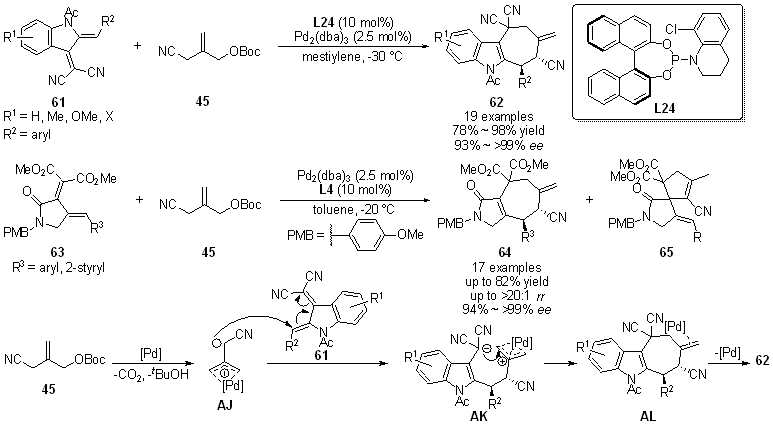
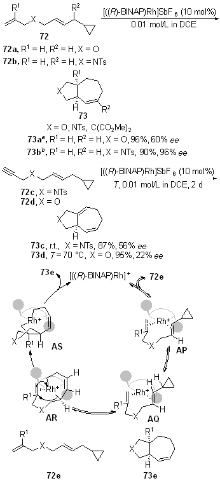
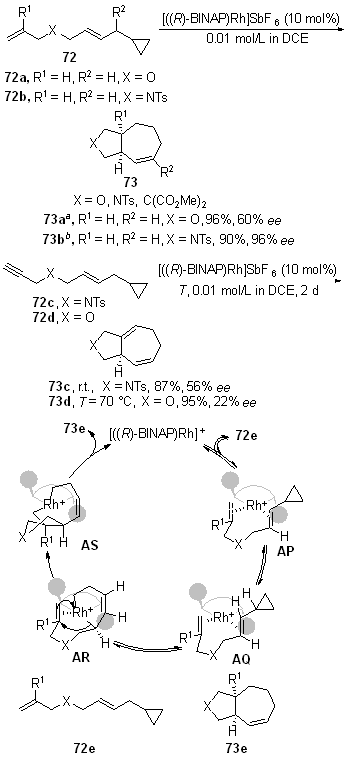
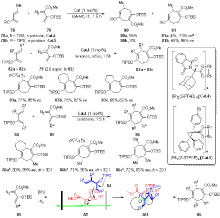
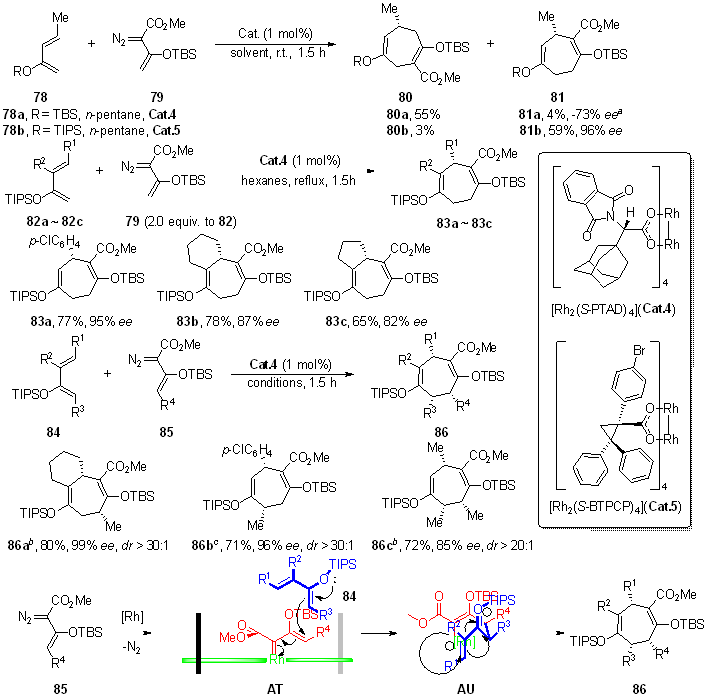
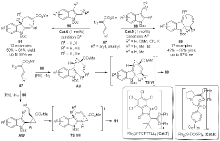
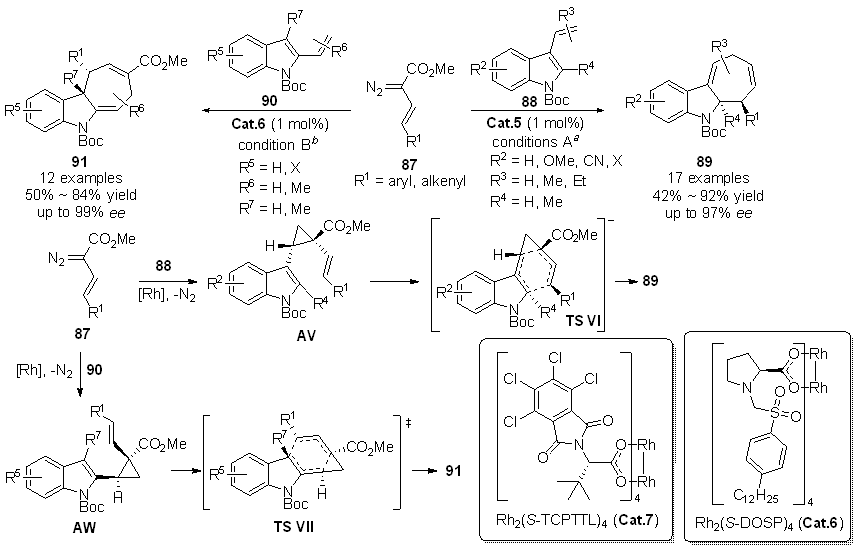
| [1] |
(a) Watthey, J. W. H.; Stanton, J. L.; Desai, M.; Babiarz, J. E.; Finn, B. M. J. Med. Chem. 1985, 28, 1511.
pmid: 24083782 |
|
(b) McDonald, L. A.; Abbanat, D. R.; Barbieri, L. R.; Bernan, V. S.; Discafani, C. M.; Greenstein, M.; Janota, K.; Korshalla, J. D.; Lassota, P.; Tischler, M.; Carter, G. T. Tetrahedron Lett. 1999, 40, 2489.
doi: 10.1016/S0040-4039(99)00242-7 pmid: 24083782 |
|
|
(c) Donnell, A. F.; Michoud, C.; Rupert, K. C.; Han, X.; Aguilar, D.; Frank, K. B.; Fretland, A. J.; Gao, L.; Goggin, B.; Hogg, J. H.; Hong, K.; Janson, C. A.; Kester, R. F.; Kong, N.; Le, K.; Li, S.; Liang, W.; Lombardo, L. J.; Lou, Y.; Lukacs, C. M.; Mischke, S.; Moliterni, J. A.; Polonskaia, A.; Schutt, A. D.; Solis, D. S.; Specian, A.; Taylor, R. T.; Weisel, M.; Remiszewski, S. W. J. Med. Chem. 2013, 56, 7772.
doi: 10.1021/jm400731m pmid: 24083782 |
|
|
(d) Xu, J. B.; Zhang, H.; Gan, L. S.; Han, Y. S.; Wainberg, M. A.; Yue, J. M. J. Am. Chem. Soc. 2014, 136, 7631.
doi: 10.1021/ja503995b pmid: 24083782 |
|
|
(e) Ni, G.; Zhang, H.; Fan, Y.-Y.; Liu, H. C.; Ding, J.; Yue, J. M. Org. Lett., 2016, 18, 1880.
doi: 10.1021/acs.orglett.6b00653 pmid: 24083782 |
|
|
(f) Ruan, Q. F.; Jiang, S. Q.; Zheng, X. Y.; Tang, Y. Q.; Yang, B.; Yi, T.; Jin, J.; Cui, H.; Zhao, Z. Chem. Commun. 2020, 56, 1517.
doi: 10.1039/C9CC09159A pmid: 24083782 |
|
| [2] |
(a) Carruthers, W. Cycloaddition Reactions in Organic Synthesis, Pergamon, Oxford, 1990, Vol. 373.
|
|
(b) Ghosez, L.; Trost, B. M. Stereocontrolled Organic Synthesis, Blackwell Science, Oxford, 1994, pp. 193-233.
|
|
|
(c) Curran, D. P. Advances in Cycloaddition, JAI Press, Greenwich, 1994, Vol. 1-3.
|
|
|
(d) Nishiwaki, N. Methods and Applications of Cycloaddition Reactions in Organic Syntheses, Wiley, Hoboken, 2014.
|
|
| [3] |
(a) Denmark, S. E.; Thorarensen, A. Chem. Rev. 1996, 96, 137.
pmid: 27018601 |
|
(b) Chopade, P. R.; Louie, J. Adv. Synth. Catal. 2006, 348, 2307.
doi: 10.1002/adsc.200600325 pmid: 27018601 |
|
|
(c) Alcaide, B.; Almendros, P.; Aragoncillo, C. Chem. Soc. Rev. 2010, 39, 783.
doi: 10.1039/b913749a pmid: 27018601 |
|
|
(d) Ljpez, F.; MascareÇas, J. L. Chem. Soc. Rev. 2014, 43, 2904.
doi: 10.1039/C4CS00024B pmid: 27018601 |
|
|
(e) Poplata, S.; Trçster, A.; Zou, Y.; Bach, T. Chem. Rev. 2016, 116, 9748.
doi: 10.1021/acs.chemrev.5b00723 pmid: 27018601 |
|
|
(f) Pellissier, H. Synthesis 2020, 52, 3837.
doi: 10.1055/s-0040-1707905 pmid: 27018601 |
|
|
(g) Trost, B. M.; Mata, G. Acc. Chem. Res. 2020, 53, 1293.
doi: 10.1021/acs.accounts.0c00152 pmid: 27018601 |
|
|
(h) Yang, C.; Yang, Z.-X.; Ding, C.-H.; Xu, B.; Hou, X. L. Chem. Rec. 2021, 21, 1442.
pmid: 27018601 |
|
| [4] |
(a) Battiste, M. A.; Pelphrey, P. M.; Wright, D. L. Chem.-Eur. J. 2006, 12, 3438.
pmid: 16402402 |
|
(b) Butenschçn, H. Angew. Chem., Int. Ed. 2008, 47, 5287.
doi: 10.1002/anie.200801738 pmid: 16402402 |
|
|
(c) Harmata, M. Chem. Commun. 2010, 46, 8886.
doi: 10.1039/c0cc03620j pmid: 16402402 |
|
| [5] |
(a) Trost, B. M.; Zuo, Z.; Schultz, J. E. Chem.-Eur. J. 2020, 26, 15354.
doi: 10.1002/chem.202002713 |
|
(b) Saranya, P. V.; Neetha, M.; Radhika, S.; Anilkumar, G. J. Heterocycl. Chem. 2021, 58, 673.
doi: 10.1002/jhet.4182 |
|
| [6] |
Hu, J.-L.; Wang, L.; Xu, H.; Xie, Z.; Tang, Y. Org. Lett. 2015, 17, 2680.
doi: 10.1021/acs.orglett.5b01077 |
| [7] |
Wei, L.; Wang, Z.-F.; Yao, L.; Qiu, G.; Tao, H.; Li, H.; Wang, C. J. Adv. Synth. Catal. 2016, 358, 3955.
doi: 10.1002/adsc.201600961 |
| [8] |
Wei, L.; Yao, L.; Wang, Z.-F.; Li, H.; Tao, H.-Y.; Wang, C. J. Adv. Synth. Catal. 2016, 358, 3748.
doi: 10.1002/adsc.201600457 |
| [9] |
Wang, Y.; Zhu, L.; Wang, M.; Xiong, J.; Chen, N.; Feng, X.; Xu, Z.; Jiang, X. Org. Lett. 2018, 20, 6506.
doi: 10.1021/acs.orglett.8b02828 |
| [10] |
Zhang, Z. J.; Zhang, L.; Geng, R. L.; Song, J.; Chen, X. H.; Gong, L. Z. Angew. Chem., Int. Ed. 2019, 58, 12190.
doi: 10.1002/anie.201907188 |
| [11] |
Zhu, X. Q.; Wang, Z. S.; Hou, B. S.; Zhang, H. W.; Deng, C.; Ye, L. W. Angew. Chem., Int. Ed. 2020, 59, 1666.
doi: 10.1002/anie.201912534 |
| [12] |
(a) Alexakis, A.; Krause, N.; Woodward, S. Copper-Catalyzed Asymmetric Synthesis, Wiley, Hoboken, 2014, pp. 1-2.
|
|
(b) Enthaler, S.; Wu, X. F. Zinc Catalysis Wiley, Hoboken, 2015, pp. 1-4.
|
|
| [13] |
Gulías, M.; Durán, J.; López, F.; Castedo, L.; Mascareñas, J. L. J. Am. Chem. Soc. 2007, 129, 11026.
doi: 10.1021/ja0756467 |
| [14] |
Verdugo, F.; Villarino, L.; Durán, J.; Gulías, M.; Mascareñas, J. L.; López, F. ACS Catal. 2018, 8, 6100.
doi: 10.1021/acscatal.8b01296 |
| [15] |
Shintani, R.; Murakami, M.; Tsuji, T.; Tanno, H.; Hayashi, T. Org. Lett. 2009, 11, 5642.
doi: 10.1021/ol902326s pmid: 19908855 |
| [16] |
(a) Guo, C.; Fleige, M.; Janssen-Müller, D.; Daniliuc, C. G.; Glorius, F. J. Am. Chem. Soc. 2016, 138, 7840.
doi: 10.1021/jacs.6b04364 |
|
(b) Guo, C.; Janssen-Müller, D.; Fleige, M.; Lerchen, A.; Daniliuc, C. G.; Glorius, F. J. Am. Chem. Soc. 2017, 139, 4443.
doi: 10.1021/jacs.7b00462 |
|
| [17] |
Singha, S.; Patra, T.; Daniliuc, C. G.; Glorius, F. J. Am. Chem. Soc. 2018, 140, 3551.
doi: 10.1021/jacs.8b00868 |
| [18] |
Jiang, F.; Yuan, F. R.; Jin, L. W.; Mei, G. J.; Shi, F. ACS Catal. 2018, 8, 10234.
doi: 10.1021/acscatal.8b03410 |
| [19] |
Cheng, Q.; Xie, J. H.; Weng, Y. C.; You, S. L. Angew. Chem., Int. Ed. 2019, 58, 5739.
doi: 10.1002/anie.201901251 |
| [20] |
Liu, Y. Z.; Wang, Z.; Huang, Z.; Zheng, X.; Yang, W. L.; Deng, W. P. Angew. Chem., Int. Ed. 2020, 59, 1238.
doi: 10.1002/anie.201909158 |
| [21] |
Trost, B. M.; Zuo, Z. Angew. Chem., Int. Ed. 2020, 59, 1243.
doi: 10.1002/anie.201911537 |
| [22] |
Kumari, P.; Liu, W.; Wang, C. J.; Dai, J.; Wang, M. X.; Yang, Q. Q.; Deng, Y. H.; Shao, Z. Chin. J. Chem. 2020, 38, 151.
doi: 10.1002/cjoc.201900430 |
| [23] |
Yang, W. L.; Huang, Z.; Liu, Y. Z.; Yu, X.; Deng, W. P. Chin. J. Chem. 2020, 38, 1571.
doi: 10.1002/cjoc.202000264 |
| [24] |
Wei, Y.; Liu, S.; Li, M. M.; Li, Y.; Lan, Y.; Lu, L. Q.; Xiao, W. J. J. Am. Chem. Soc. 2019, 141, 133.
doi: 10.1021/jacs.8b12095 pmid: 30540187 |
| [25] |
Li, M. M.; Xiong, Q.; Qu, B. L.; Xiao, Y. Q.; Lan, Y.; Lu, L. Q.; Xiao, W. J. Angew. Chem., Int. Ed. 2020, 59, 17429.
doi: 10.1002/anie.202006366 |
| [26] |
Zheng, X.; Sun, H.; Yang, W. L.; Deng, W. P. Sci. China: Chem. 2020, 63, 911.
|
| [27] |
Liu, Y. Z.; Wang, Z.; Huang, Z.; Yang, W. L.; Deng, W. P. Org. Lett. 2021, 23, 948.
doi: 10.1021/acs.orglett.0c04146 pmid: 33481618 |
| [28] |
Gao, Y. F.; Zhang, X. G.; Zhang, X. Y.; Miao, Z. W. Org. Lett. 2021, 23, 2415.
doi: 10.1021/acs.orglett.1c00073 |
| [29] |
Tsuji, J. Palladium Reagents and Catalysts, Wiley, Hoboken, 2004, pp. 1-26.
|
| [30] |
Wender, P. A.; Haustedt, L. O.; Lim, J.; Love, J. A.; Williams, T. J.; Yoon, J. Y. J. Am. Chem. Soc. 2006, 128, 6302.
pmid: 16683779 |
| [31] |
Shintani, R.; Nakatsu, H.; Takatsu, K.; Hayashi, T. Chem.-Eur. J. 2009, 15, 8692.
doi: 10.1002/chem.200901463 pmid: 19637169 |
| [32] |
Feng, J. J.; Lin, T. Y.; Wu, H. H.; Zhang, J. L. J. Am. Chem. Soc. 2015, 137, 3787.
doi: 10.1021/jacs.5b01305 |
| [33] |
Guzmán, P. E.; Lian, Y.; Davies, H. M. L. Angew. Chem., Int. Ed. 2014, 53, 13083.
doi: 10.1002/anie.201406440 |
| [34] |
Xu, G.; Chen, L.; Sun, J. Org. Lett. 2018, 20, 3408.
doi: 10.1021/acs.orglett.8b01353 |
| [35] |
Jia, Z. J.; Shan, G.; Daniliuc, C. G.; Antonchick, A. P.; Waldmann, H. Angew. Chem., Int. Ed. 2018, 57, 14493.
doi: 10.1002/anie.201712882 |
| [36] |
(a) Huang, L.; Dai, L.-X.; You, S.-L.. J. Am. Chem. Soc. 2016, 138, 5793.
doi: 10.1021/jacs.6b02678 pmid: 27093370 |
|
(b) Huang, L.; Cai, Y.; Zheng, C.; Dai, L. X.; You, S. L. Angew. Chem., Int. Ed. 2017, 56, 10545.
doi: 10.1002/anie.201705068 pmid: 27093370 |
|
| [37] |
Chen, Z. C.; Chen, Z.; Yang, Z. H.; Guo, L.; Du, W.; Chen, Y. C. Angew. Chem., Int. Ed. 2019, 58, 15021.
doi: 10.1002/anie.201907797 |
| [38] |
Yang, W. L.; Ni, T.; Deng, W. P. Org. Lett. 2021, 23, 588.
doi: 10.1021/acs.orglett.0c04132 |
| [39] |
Li, Y. Y.; Li, S.; Fan, T.; Zhang, Z. J.; Song, J.; Gong, L. Z. ACS Catal. 2021, 11, 14388.
doi: 10.1021/acscatal.1c04541 |
| [40] |
Alonso, I.; Faustino, H.; López, F.; Mascareñas, J. L. Angew. Chem., Int. Ed. 2011, 50, 11496.
doi: 10.1002/anie.201105815 |
| [41] |
Kardile, R. D.; Chao, T. H.; Cheng, M. J.; Liu, R. S. Angew. Chem., Int. Ed. 2020, 59, 10396.
doi: 10.1002/anie.202001854 |
| [1] | Huakun Wang, Xiaolong Ren, Yining Xuan. Study of the Halide Salt Catalyzed [3+2] Cycloaddition of α,β-Epoxy Carboxylate with Isocyanate [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 251-258. |
| [2] | Hongqiong Zhao, Miao Yu, Dongxue Song, Qi Jia, Yingjie Liu, Yubin Ji, Ying Xu. Progress on Decarboxylation and Hydroxylation of Carboxylic Acids [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 70-84. |
| [3] | Shihang Yu, Jiawei Liu, Biyu An, Qinghua Bian, Min Wang, Jiangchun Zhong. Asymmetric Synthesis of the Contact Sex Pheromone of Neoclytus acuminatus acuminatus (Fabricius) [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 301-308. |
| [4] | Yuchao Wang, Jinbiao Liu, Zhitao He. Palladium-Catalyzed Asymmetric Hydrofunctionalizations of Conjugated Dienes [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2614-2627. |
| [5] | Xiaojing Hu, Feixiang Guo, Runqing Zhu, Bingqi Zhou, Tao Zhang, Lizhen Fang. Synthesis of p-Alkoxy Phenol and Its Application after Dearomatization [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2239-2244. |
| [6] | Tingyu Song, Ran Li, Lihua Huang, Shikun Jia, Guangjian Mei. Catalytic Asymmetric Synthesis of N—N Atropisomers [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1977-1990. |
| [7] | Cheng Luo, Yanli Yin, Zhiyong Jiang. Recent Advances in Asymmetric Synthesis of P-Chiral Phosphine Oxides [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1963-1976. |
| [8] | Guangli Xu, Jing Xu, Haidong Xu, Xiang Cui, Xingzhong Shu. Research Progress of Transition Metal Catalyzed Synthesis of 1,3- Conjugated Diene Compounds from Alkenes and Alkynes [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1899-1933. |
| [9] | Hairui Jia, Zaozao Qiu. Recent Advances in Transition Metal-Catalyzed B—H Bond Activation for Synthesis of o-Carborane Derivatives with B—Heteroatom Bond [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1045-1068. |
| [10] | Kongchuan Wu, Kaihong Lu, Jianbin Lin, Huijun Zhang. Research Progress in Ortho-C—H Bond Functionalization of Rylene Diimides [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1000-1011. |
| [11] | Ling Meng, Jun Wang. Research Progress on Synthesis of Thioflavonoids [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 873-891. |
| [12] | Huaiyuan Zhang, Nuo Xu, Rongping Tang, Xingli Shi. Recent Advances in Asymmetric Dearomatization Reactions Induced by Chiral Hypervalent Iodine Reagents [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3784-3805. |
| [13] | Min Liu, Liping Qi, Dongbing Zhao. Recent Advances in Transition Metal-Catalyzed C—Si Bond Cleavage of Silacyclobutanes [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3508-3525. |
| [14] | Liu-Yang Pu, Zhiyue Li, Limin Li, Yucui Ma, Min Ma, Shengquan Hu, Zhengzhi Wu. Asymmetric Synthesis of (–)-Colchicine and Its Natural Analog (–)-N-Acetylcolchicine Methyl Ether [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 313-319. |
| [15] | Donghan Liu, Xihang Lu, Zhangmengjie Chai, Haoqi Yang, Yulin Sun, Fuchao Yu. Progress in Construction of 2H-Pyrrol-2-ones Skeleton [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 57-73. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||