Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (7): 1883-1903.DOI: 10.6023/cjoc202203006 Previous Articles Next Articles
ACCOUNT
高润烨a,b, 左玲玲b,e,*( ), 王芳b,e, 李传莹a,*(
), 王芳b,e, 李传莹a,*( ), 蒋华江b, 李品华c,d,*(
), 蒋华江b, 李品华c,d,*( ), 王磊b,c,e,*(
), 王磊b,c,e,*( )
)
收稿日期:2022-03-01
修回日期:2022-03-26
发布日期:2022-08-09
通讯作者:
左玲玲, 李传莹, 李品华, 王磊
基金资助:
Runye Gaoa,b, Lingling Zuob,e( ), Fang Wangb,e, Chuanying Lia(
), Fang Wangb,e, Chuanying Lia( ), Huajiang Jiangb, Pinhua Lic,d(
), Huajiang Jiangb, Pinhua Lic,d( ), Lei Wangb,c,e(
), Lei Wangb,c,e( )
)
Received:2022-03-01
Revised:2022-03-26
Published:2022-08-09
Contact:
Lingling Zuo, Chuanying Li, Pinhua Li, Lei Wang
Supported by:Share
Runye Gao, Lingling Zuo, Fang Wang, Chuanying Li, Huajiang Jiang, Pinhua Li, Lei Wang. Recent Advances in Controllable Organic Reactions Induced by Visible Light without External Photocatalyst[J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 1883-1903.
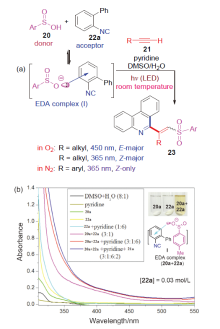
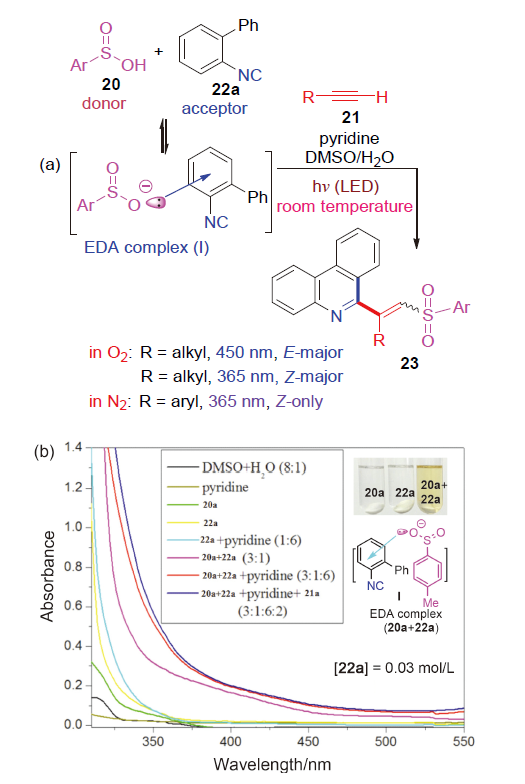

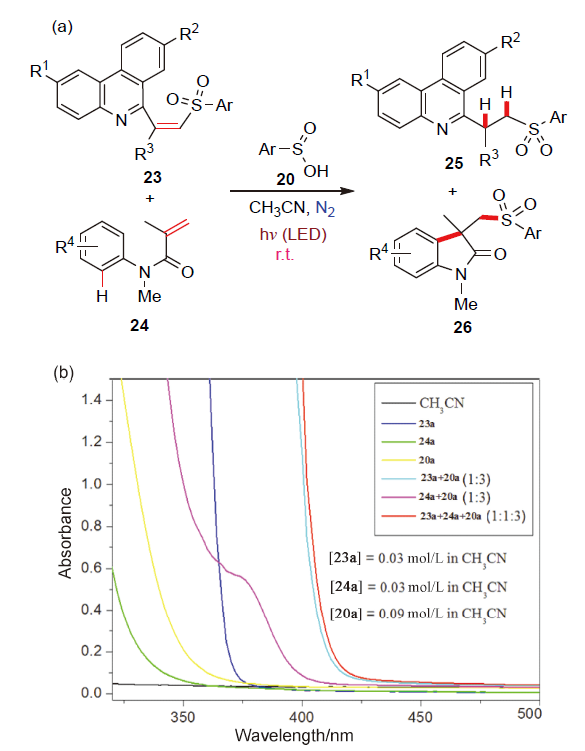


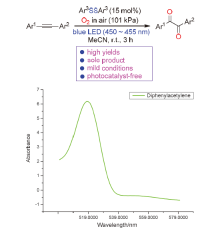

| [1] |
Ciamician, G. Science 1912, 36, 385.
pmid: 17836492 |
| [2] |
(a) Norrish, R. G. W.; Bamford, C. H. Nature 1936, 138, 1016.
doi: 10.1038/1381016a0 |
|
(b) Norrish, R. G. W.; Bamford, C. H. Nature 1937, 140, 195.
|
|
|
(c) Yang, N. C.; Yang, D.-D. H. J. Am. Chem. Soc. 1958, 80, 2913.
|
|
| [3] |
Nicewicz, D. A.; MacMillan, D. W. C. Science 2008, 322, 77.
doi: 10.1126/science.1161976 pmid: 18772399 |
| [4] |
(a) Chen, Y.-Y.; Lu, L.-Q.; Yu, D.-G.; Zhu, C.-J.; Xiao, W.-J. Sci. China Chem. 2019, 62, 24.
doi: 10.1007/s11426-018-9399-2 pmid: 18772399 |
|
(b) Yu, X.-Y.; Chen, J.-R.; Xiao, W.-J. Chem. Rev. 2021, 121, 506.
doi: 10.1021/acs.chemrev.0c00030 pmid: 18772399 |
|
|
(c) Yu, X.-Y.; Zhao, Q.-Q.; Chen, J.; Xiao, W.-J.; Chen, J.-R. Acc. Chem. Res. 2020, 53, 1066
doi: 10.1021/acs.accounts.0c00090 pmid: 18772399 |
|
|
(d) Lei, T.; Zhou, C.; Huang, M.-Y.; Zhao, L.-M.; Yang, B.; Ye, C.; Xiao, H.; Meng, Q.-Y.; Ramamurthy, V.; Tung, C.-H.; Wu, L.-Z. Angew. Chem., Int. Ed. 2017, 56, 15407.
doi: 10.1002/anie.201708559 pmid: 18772399 |
|
|
(e) Nicewicz, D. A.; MacMillan, D. W. C. Science 2008, 322, 77.
doi: 10.1126/science.1161976 pmid: 18772399 |
|
|
(f) Genzink, M. J.; Kidd, J. B.; Swords, W. B.; Yoon, T. P. Chem. Rev. 2022, 122, 1654.
doi: 10.1021/acs.chemrev.1c00467 pmid: 18772399 |
|
|
(g) Allen, A. R.; Noten, E. A.; Stephenson, C. R. J. Chem. Rev. 2022, 122, 2695.
doi: 10.1021/acs.chemrev.1c00388 pmid: 18772399 |
|
|
(h) Wang, H.; Gao, X.; Lv, Z.; Abdelilah, T.; Lei, A. Chem. Rev. 2019, 119, 6769.
doi: 10.1021/acs.chemrev.9b00045 pmid: 18772399 |
|
|
(i) Zhang, M.; Xie, J.; Zhu, C. Nat. Commun. 2018, 9, 3517.
doi: 10.1038/s41467-018-06019-1 pmid: 18772399 |
|
| [5] |
Skubi, K. L.; Blum, T. R.; Yoon, T. P. Chem. Rev. 2016, 116, 10035.
doi: 10.1021/acs.chemrev.6b00018 |
| [6] |
(a) Wei, Y.; Zhou, Q.-Q.; Tan, F.; Lu, L.-Q.; Xiao, W.-J. Synthesis 2019, 51, 3021.
doi: 10.1055/s-0037-1611812 |
|
(b) Liu, W.; Li, C.-J. Synlett 2017, 28, 2714.
doi: 10.1055/s-0036-1590900 |
|
|
(c) Li, L.; Mu, X.; Liu, W.; Wang, Y.; Mi, Z.; Li, C.-J. J. Am. Chem. Soc. 2016, 138, 5809.
doi: 10.1021/jacs.6b02782 |
|
|
(d) Masuda, Y.; Ishida, N.; Murakami, M. J. Am. Chem. Soc. 2015, 137, 14063.
doi: 10.1021/jacs.5b10032 |
|
|
(e) Sato, Y.; Kawaguchi, S.; Nomoto, A.; Ogawa, A. Angew. Chem., Int. Ed. 2016, 55, 9700.
doi: 10.1002/anie.201603860 |
|
| [7] |
Yang, W.; Yang, S.; Li, P.; Wang, L. Chem. Commun. 2015, 51, 7520.
doi: 10.1039/C5CC00878F |
| [8] |
Sun, M.; Wang, L.; Li, L.; Sun, M.; Huo, J.; Li, P. Org. Chem. Front. 2021, 8, 4230.
doi: 10.1039/D1QO00592H |
| [9] |
Wang, B.; Zou, L.; Wang, L.; Sun, M.; Li, P. Chin. Chem. Lett. 2021, 32, 1229.
doi: 10.1016/j.cclet.2020.08.013 |
| [10] |
Yang, S.; Wang, L.; Wang, L.; Li, H. J. Org. Chem. 2020, 85, 564.
doi: 10.1021/acs.joc.9b02646 |
| [11] |
(a) Yang, S.; Li, P.; Wang, Z.; Wang, L. Org. Lett. 2017, 19, 3386.
doi: 10.1021/acs.orglett.7b01230 |
|
(b) Ji, W.; Li, P.; Yang, S.; Wang, L. Chem. Commun. 2017, 53, 8482.
doi: 10.1039/C7CC03693K |
|
| [12] |
Zhao, L.; Li, P.; Xie, X.; Wang, L. Org. Chem. Front. 2018, 5, 1689.
doi: 10.1039/C8QO00229K |
| [13] |
Ren, Y.; Meng, L.-G.; Peng, T.; Wang, L. Org. Lett. 2018, 20, 4430.
doi: 10.1021/acs.orglett.8b01714 |
| [14] |
Zhang, Y.; Chen, W.; Jia, X.; Wang, L.; Li, P. Chem. Commun. 2019, 55, 2785.
doi: 10.1039/C8CC10235J |
| [15] |
Zhao, L.; Li, P.; Xie, X.; Wang, L. Org. Chem. Front. 2019, 6, 87.
doi: 10.1039/C8QO01079J |
| [16] |
Wang, Z.; Wang, L.; Wang, Z.; Li, P.; Zhang, Y. Chin. Chem. Lett. 2021, 32, 429.
doi: 10.1016/j.cclet.2020.02.022 |
| [17] |
Zhou, C.; Li, P.; Zhu, X.; Wang, L. Org. Lett. 2015, 17, 6198.
doi: 10.1021/acs.orglett.5b03192 |
| [18] |
Xu, N.; Li, P.; Xie, Z.; Wang, L. Chem. Eur. J. 2016, 22, 2236.
doi: 10.1002/chem.201504530 |
| [19] |
Twilton, J.; Le, C.; Zhang, P.; Shaw, M. H.; Evans, R. W.; MacMillan, D. W. C. Nat. Rev. Chem. 2017, 1, 0052.
doi: 10.1038/s41570-017-0052 |
| [20] |
Zou, L.; Li, P.; Wang, B.; Wang, L. Chem. Commun. 2019, 55, 3737.
doi: 10.1039/C9CC01014A |
| [21] |
(a) Sun, M.; Wang, L.; Zhao, L.; Wang, Z.; Li, P. ChemCatChem 2020, 12, 5261.
doi: 10.1002/cctc.202000459 |
|
(b) Xu, J.; Zhang, H.; Zhao, J.; Ni, Z.; Zhang, P.; Shi, B.-F.; Li, W. Green Chem. 2021, 23, 2123.
doi: 10.1039/D0GC04235H |
|
|
(c) Huang, L.; Xu, J.; He, L.; Liang, C.; Ouyang, Y.; Yu, Y.; Li, W.; Zhang, P. Chin. Chem. Lett. 2021, 32, 3627.
doi: 10.1016/j.cclet.2021.04.016 |
|
|
(d) He, L.; Liang, C.; Ouyang, Y.; Li, L.; Guo, Y.; Zhang, P.; Li, W. Org. Biomol. Chem. 2022, 20, 790.
doi: 10.1039/D1OB02249K |
|
|
(e) Xu, J.; He, L.; Liang, C.; Yue, X.; Ouyang, Y.; Zhang, P. ACS Sustainable Chem. Eng. 2021, 9, 13663.
doi: 10.1021/acssuschemeng.1c05237 |
|
| [22] |
Zou, L.; Wang, L.; Sun, L.; Xie, X.; Li, P. Chem. Commun. 2020, 56, 7933.
doi: 10.1039/D0CC02471F |
| [23] |
Gao, Y.; Zhao, L. Xiang, T.; Li, P.; Wang, L. RSC Adv. 2020, 10, 10559.
doi: 10.1039/D0RA02059A |
| [24] |
Zhang, W.; Bu, J.; Wang, L.; Li, P.; Li, H. Org. Chem. Front. 2021, 8, 5045.
doi: 10.1039/D1QO00739D |
| [25] |
(a) Xie, X.; Pan, H.; Zhou, T.-P.; Han, M.-Y.; Wang, L.; Geng, X.; Ma, Y.; Liao, R.-Z.; Wang, Z.-M.; Yang, J.; Li, P. Org. Chem. Front. 2021, 8, 5872.
doi: 10.1039/D1QO01017D |
|
(b) Xie, X.; Wang, L.; Zhou, Q.; Ma, Y.; Wang, Z.-M.; Li, P. Chin. Chem. Lett. 2022, 10.1016/j.cclet.2022.03.084.
doi: 10.1016/j.cclet.2022.03.084 |
|
| [26] |
Arceo, E.; Jurberg, I. D.; Álvarez-Fernaández, A.; Melchiorre, P. Nat. Chem. 2013, 5, 750.
doi: 10.1038/nchem.1727 |
| [27] |
Nappi, M.; Bergonzini, G.; Melchiorre, P. Angew. Chem., Int. Ed. 2014, 53, 4921.
doi: 10.1002/anie.201402008 |
| [28] |
Kandukuri, S. R.; Bahamonde, A.; Chatterjee, I.; Jurberg, I. D.; Escudero-Adán, E. C.; Melchiorre, P. Angew. Chem., Int. Ed. 2015, 54, 1485.
doi: 10.1002/anie.201409529 |
| [29] |
Bahamonde, A.; Melchiorre, P. J. Am. Chem. Soc. 2016, 138, 8019.
doi: 10.1021/jacs.6b04871 pmid: 27267587 |
| [30] |
Li, Y.; Miao, T.; Li, P.; Wang, L. Org. Lett. 2018, 20, 1735.
doi: 10.1021/acs.orglett.8b00171 |
| [31] |
Li, Y.; Ma, F.; Li, P.; Miao, T.; Wang, L. Adv. Synth. Catal. 2019, 361, 1606.
doi: 10.1002/adsc.201801521 |
| [32] |
Shi, W.; Ma, F.; Li, P.; Wang, L.; Miao, T. J. Org. Chem. 2020, 85, 13808.
doi: 10.1021/acs.joc.0c01916 |
| [33] |
(a) Yao, L.; Zhu, D.; Wang, L.; Liu, J.; Zhang, Y.; Li, P. Chin. Chem. Lett. 2021, 32, 4033.
doi: 10.1016/j.cclet.2021.06.005 |
|
(b) Zeng, F.-L.; Xie, K.-C.; Liu, Y.-T.; Wang, H.; Yin, P.-C.; Qu, L.-B.; Chen, X.-L.; Yu, B. Green Chem. 2022, 24, 1732.
doi: 10.1039/D1GC04218A |
|
|
(c) Li, X.; Cui, W.; Deng, Q.; Song, X.; Lv, J.; Yang, D. Green Chem. 2022, 24, 1302.
doi: 10.1039/D1GC04184C |
|
|
(d) Tong, J.; Li, H.; Zhu, Y.; Liu, P.; Sun, P. Green Chem. 2022, 24, 1995.
doi: 10.1039/D1GC04703E |
|
| [34] |
(a) Tan, H.; Li, H.; Ji, W.; Wang, L. Angew. Chem., Int. Ed. 2015, 54, 8374.
doi: 10.1002/anie.201503479 |
|
(b) Huang, H.; Zhang, G.; Chen, Y. Angew. Chem., Int. Ed. 2015, 54, 7872.
doi: 10.1002/anie.201502369 |
|
| [35] |
Ji, W.; Tan, H.; Wang, M.; Li, P.; Wang, L. Chem. Commun. 2016, 52, 1462.
doi: 10.1039/C5CC08253F |
| [36] |
Yang, S.; Tan, H.; Ji, W.; Zhang, X.; Li, P.; Wang, L. Adv. Synth. Catal. 2017, 359, 443.
doi: 10.1002/adsc.201600721 |
| [37] |
Ni, K.; Meng, L.-G.; Wang, K.; Wang, L. Org. Lett. 2018, 20, 2245.
doi: 10.1021/acs.orglett.8b00586 |
| [38] |
Ni, K.; Meng, L.-G.; Ruan, H.; Wang, L. Chem. Commun. 2019, 55, 8438.
doi: 10.1039/C9CC04090K |
| [39] |
Xie, X.; Liu, J.; Wang, L.; Wang, M. Eur. J. Org. Chem. 2020, 1534.
|
| [40] |
Zhu, X.; Li, P.; Shi, Q.; Wang, L. Green Chem. 2016, 18, 6373.
doi: 10.1039/C6GC01487A |
| [41] |
Shi, Q.; Li, P.; Zhang, Y.; Wang, L. Org. Chem. Front. 2017, 4, 1322.
doi: 10.1039/C7QO00152E |
| [42] |
Ye, R.; Ruan, H.; Xu, H.; Li, Z.; Meng, L.-G.; Wang, L. Org. Chem. Front. 2021, 8, 5345.
doi: 10.1039/D1QO01082D |
| [43] |
Ruan, H.; Meng, L.-G.; Zhu, L.; Wang, L. Adv. Synth. Catal. 2019, 361, 3217.
doi: 10.1002/adsc.201900140 |
| [44] |
Wang, Z.; Li, X.; Wang, L.; Li, P. Tetrahedron 2019, 75, 1044.
doi: 10.1016/j.tet.2019.01.013 |
| [45] |
Wang, Z.; Wang, L.; Wang, Z.; Li, P. Asian J. Org. Chem. 2019, 8, 1448.
doi: 10.1002/ajoc.201900333 |
| [46] |
Ni, S.; Cao, J.; Mei, H.; Han, J.; Li, S.; Pan, Y. Green Chem. 2016, 18, 3935.
doi: 10.1039/C6GC01027J |
| [47] |
Zhao, B.; Zhang, Z.; Ge, Y.; Li, P.; Miao, T.; Wang, L. Org. Chem. Front. 2021, 8, 975.
doi: 10.1039/D0QO01487G |
| [48] |
Xie, Z.; Li, P.; Hu, Y.; Xu, N.; Wang, L. Org. Biomol. Chem. 2017, 15, 4205.
doi: 10.1039/C7OB00779E |
| [49] |
Xu, N.; Zhang, Y.; Chen, W.; Li, P.; Wang, L. Adv. Synth. Catal. 2018, 360, 1199.
doi: 10.1002/adsc.201701548 |
| [50] |
Mei, Y.; Zhao, L.; Liu, Q.; Ruan, S.; Wang, L.; Li, P. Green Chem. 2020, 22, 2270.
doi: 10.1039/D0GC00009D |
| [1] | Luyao Li, Zhongwen He, Zhenguo Zhang, Zhenhua Jia, Teck-Peng Loh. Application of Triaryl Carbenium in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 421-437. |
| [2] | Qianfan Zhao, Yongzheng Chen, Shiming Zhang. Application and Mechanism Study of Carbon-Based Metal-Free Catalysts in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 137-147. |
| [3] | Ran Zhou, Chunmei Yuan, Tao Zhang, Piao Mao, Yi Liu, Kaini Meng, Hui Xin, Wei Xue. Design, Synthesis and Bioactivity of Chalcone Derivative Containing Quinazolinone [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3196-3209. |
| [4] | Yang Li, Jinding Yuan, Di Zhao. Deep Eutectic Solvent of 1,3-Dimethylurea/L-(+)-Tartaric Acid for the Green Synthesis of (E)-2-Styrylquinoline-3-carboxylic Acid Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3268-3276. |
| [5] | Guangli Xu, Jing Xu, Haidong Xu, Xiang Cui, Xingzhong Shu. Research Progress of Transition Metal Catalyzed Synthesis of 1,3- Conjugated Diene Compounds from Alkenes and Alkynes [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1899-1933. |
| [6] | Qian Dou, Taimin Wang, Lijing Fang, Hongbin Zhai, Bin Cheng. Recent Development of Photoinduced Iron-Catalysis in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1386-1415. |
| [7] | Shiquan Gao, Chuangjun Liu, Junfeng Yang, Junliang Zhang. Cobalt-Catalyzed Electrochemical Reductive Coupling of Alkynes and Alkenes [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1559-1565. |
| [8] | Linsheng Bai, Peng Hong, Anguo Ying. Research Progress of Functional Polyacrylonitrile Fiber in Promoting Organic Reaction [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1241-1270. |
| [9] | Baichuan Mo, Chunxia Chen, Jinsong Peng. Research Progress in Application of Lignin and Its Derivatives Supported Metal Catalysts in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1215-1240. |
| [10] | Jian Ji, Jinhua Liu, Cong Guan, Xuwen Chen, Yun Zhao, Shunying Liu. High Regioselective Synthesis of N2-Substituted-1,2,3-triazole via N-Sulfonyl-1,2,3-triazole Coupling with Alcohol Catalyzed by in-situ Generated Sulfonic Acid [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1168-1176. |
| [11] | Biao Ma, Miaomiao Zhang, Zhanyu Li, Jinsong Peng, Chunxia Chen. Recent Advance of Transition Metal-Free Catalyzed Suzuki-Type Cross Coupling Reaction [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 455-470. |
| [12] | Shuo Li, Mingliang Wang, Laiyun Zhou, Lanzhi Wang. Magnetic Nano-Supported p-Toluenesulfonic Acid Catalyzed Synthesis of Fused Polycyclic 1,5-Benzoxazepines via Domino Reactions [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3977-3988. |
| [13] | Saimi Naibijiang, Lei Zhang, Shalamu Maidina, Jing Zeng, Abudu Rexit Abulikemu. Green Synthesis of Thiosulfonates and Sulfonyl Halides [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 236-243. |
| [14] | Silin Chen, Yunhui Yang, Chao Chen, Congyang Wang. Advances in Transition-Metal-Catalyzed Keto Carbonyl-Directed C—H Bond Functionalization Reactions [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 1-16. |
| [15] | Zhentao Pan, Tong Liu, Yongmin Ma, Jianbo Yan, Ya-Jun Wang. Construction of Quinazolin(thi)ones by Brønsted Acid/Visible-Light Photoredox Relay Catalysis [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2823-2831. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||