Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (11): 3843-3852.DOI: 10.6023/cjoc202205008 Previous Articles Next Articles
ARTICLES
应安国a, 白林盛a, 侯海亮b, 许松林b, 鲁小彤a, 王丽敏a,*( )
)
收稿日期:2022-05-05
修回日期:2022-06-10
发布日期:2022-07-05
通讯作者:
王丽敏
基金资助:
Anguo Yinga, Linsheng Baia, Hailiang Houb, Songlin Xub, Xiaotong Lua, Limin Wanga( )
)
Received:2022-05-05
Revised:2022-06-10
Published:2022-07-05
Contact:
Limin Wang
Supported by:Share
Anguo Ying, Linsheng Bai, Hailiang Hou, Songlin Xu, Xiaotong Lu, Limin Wang. Research on Thia-Michael Addition Tandem Reactions Catalyzed by AlCl3@MNPs[J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3843-3852.
| Entry | Catalyst (g) | Solvent | Time/h | Yieldb/% |
|---|---|---|---|---|
| 1 | AlCl3@MNPs (0.20) | Ethanol | 5 | 97 |
| 2 | AlCl3 (0.04) | Ethanol | 5 | 95 |
| 3 | FeCl3 (0.04) | Ethanol | 5 | 53 |
| 4 | ZnCl2 (0.04) | Ethanol | 5 | 38 |
| 3 | MNPs (0.16) | Ethanol | 10 | Trace |
| 4 | — | Ethanol | 14 | Trace |
| 5 | AlCl3@MNPs (0.20) | Methanol | 5 | 93 |
| 6 | AlCl3@MNPs (0.2) | CH2Cl2 | 6 | 82 |
| 7 | AlCl3@MNPs (0.2) | Toluene | 6 | 84 |
| 8 | AlCl3@MNPs (0.01) | Ethanol | 8 | 30 |
| 9 | AlCl3@MNPs (0.05) | Ethanol | 6 | 58 |
| 10 | AlCl3@MNPs (0.10) | Ethanol | 5 | 76 |
| 11 | AlCl3@MNPs (0.25) | Ethanol | 5 | 96 |
| Entry | Catalyst (g) | Solvent | Time/h | Yieldb/% |
|---|---|---|---|---|
| 1 | AlCl3@MNPs (0.20) | Ethanol | 5 | 97 |
| 2 | AlCl3 (0.04) | Ethanol | 5 | 95 |
| 3 | FeCl3 (0.04) | Ethanol | 5 | 53 |
| 4 | ZnCl2 (0.04) | Ethanol | 5 | 38 |
| 3 | MNPs (0.16) | Ethanol | 10 | Trace |
| 4 | — | Ethanol | 14 | Trace |
| 5 | AlCl3@MNPs (0.20) | Methanol | 5 | 93 |
| 6 | AlCl3@MNPs (0.2) | CH2Cl2 | 6 | 82 |
| 7 | AlCl3@MNPs (0.2) | Toluene | 6 | 84 |
| 8 | AlCl3@MNPs (0.01) | Ethanol | 8 | 30 |
| 9 | AlCl3@MNPs (0.05) | Ethanol | 6 | 58 |
| 10 | AlCl3@MNPs (0.10) | Ethanol | 5 | 76 |
| 11 | AlCl3@MNPs (0.25) | Ethanol | 5 | 96 |
| Entry | R1 | R2 | Time/h | 8 | Yieldb/% |
|---|---|---|---|---|---|
| 1 | 4-Me-piperidyl | NH2 | 5 | 8a | 97 |
| 2 | 4-Me-piperidyl | N(CH3)2 | 6 | 8b | 90 |
| 3 | 4-Me-piperidyl | NHCH(CH3)2 | 6 | 8c | 92 |
| 4 | 4-Me-piperidyl | Morpholinyl | 6 | 8d | 93 |
| 5 | 4-Me-piperidyl | NHCH2OH | 6 | 8e | 95 |
| 6 | 4-Me-piperidyl | NHC(CH3)3 | 7 | 8f | 68 |
| 7 | 4-Me-piperidyl | NHC(CH3)2- CH2COCH3 | 8 | 8g | 59 |
| 8 | 4-Me-piperidyl | OCH3 | 5 | 8h | 99 |
| 9 | Piperidyl | NH2 | 5 | 8i | 86 |
| 10 | Piperidyl | NHCH2OH | 6 | 8j | 90 |
| 11 | Piperidyl | Morpholinyl | 6 | 8k | 86 |
| 12 | Piperidyl | NHCH(CH3)2 | 6 | 8l | 87 |
| 13 | Piperidyl | N(CH3)2 | 5 | 8m | 87 |
| 14 | Morpholinyl | Morpholinyl | 6 | 8n | 80 |
| 15 | Morpholinyl | N(CH3)2 | 6 | 8o | 90 |
| 16 | Morpholinyl | OCH3 | 5 | 8p | 86 |
| 17 | Pyrrolidyl | NHCH(CH3)2 | 6 | 8q | 95 |
| 18 | Pyrrolidyl | NHCH2OH | 6 | 8r | 95 |
| 19 | Pyrrolidyl | Morpholinyl | 6 | 8s | 85 |
| 20 | Pyrrolidyl | N(CH3)2 | 6 | 8t | 84 |
| Entry | R1 | R2 | Time/h | 8 | Yieldb/% |
|---|---|---|---|---|---|
| 1 | 4-Me-piperidyl | NH2 | 5 | 8a | 97 |
| 2 | 4-Me-piperidyl | N(CH3)2 | 6 | 8b | 90 |
| 3 | 4-Me-piperidyl | NHCH(CH3)2 | 6 | 8c | 92 |
| 4 | 4-Me-piperidyl | Morpholinyl | 6 | 8d | 93 |
| 5 | 4-Me-piperidyl | NHCH2OH | 6 | 8e | 95 |
| 6 | 4-Me-piperidyl | NHC(CH3)3 | 7 | 8f | 68 |
| 7 | 4-Me-piperidyl | NHC(CH3)2- CH2COCH3 | 8 | 8g | 59 |
| 8 | 4-Me-piperidyl | OCH3 | 5 | 8h | 99 |
| 9 | Piperidyl | NH2 | 5 | 8i | 86 |
| 10 | Piperidyl | NHCH2OH | 6 | 8j | 90 |
| 11 | Piperidyl | Morpholinyl | 6 | 8k | 86 |
| 12 | Piperidyl | NHCH(CH3)2 | 6 | 8l | 87 |
| 13 | Piperidyl | N(CH3)2 | 5 | 8m | 87 |
| 14 | Morpholinyl | Morpholinyl | 6 | 8n | 80 |
| 15 | Morpholinyl | N(CH3)2 | 6 | 8o | 90 |
| 16 | Morpholinyl | OCH3 | 5 | 8p | 86 |
| 17 | Pyrrolidyl | NHCH(CH3)2 | 6 | 8q | 95 |
| 18 | Pyrrolidyl | NHCH2OH | 6 | 8r | 95 |
| 19 | Pyrrolidyl | Morpholinyl | 6 | 8s | 85 |
| 20 | Pyrrolidyl | N(CH3)2 | 6 | 8t | 84 |
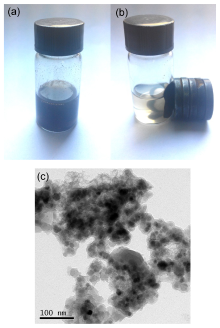

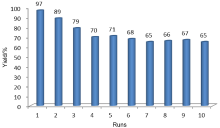
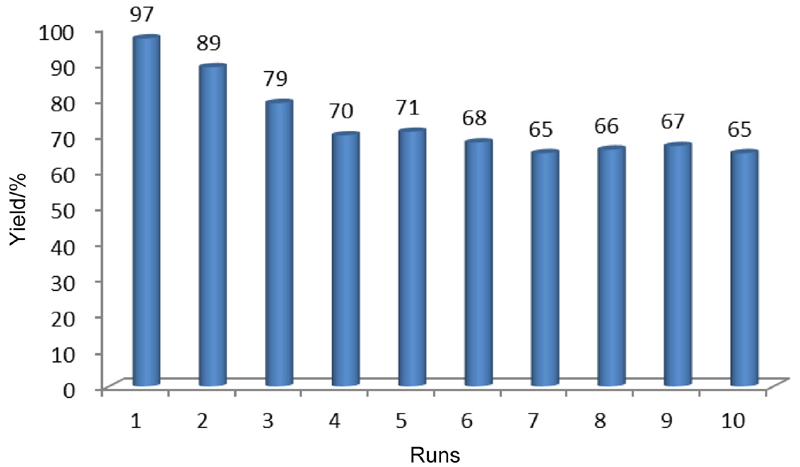
| [1] |
Dickinsonl, W. B.; Vaupotic, M. P. US 4039550, 1977.
|
| [2] |
Bergman, R. W.; Smith, H. A. US 4618461, 1986.
|
| [3] |
Mashhadizadeh, M. H.; Talemi, R, P.; Shockravi, A.; Kamali, M. Anal. Methods 2012, 4, 742.
doi: 10.1039/c2ay05559g |
| [4] |
Meng, X. M.; Liu, L.; Hu, H. Y.; Zhu, M. H.; Wang, M. X.; Shi, J.; Guo, Q. X. Tetrahedron Lett. 2006, 47, 7961.
doi: 10.1016/j.tetlet.2006.08.127 |
| [5] |
Kondoh, A.; Hirozane, T.; Terada, M. Chem. Eur. J. 2022, e202201240.
|
| [6] |
Xie, Q. X.; Liu, L. X.; Zhu, Z. H.; Yu, C. B.; Zhou, Y. G. J. Org. Chem. 2022, 87, 7521.
doi: 10.1021/acs.joc.2c00418 |
| [7] |
Khan, A. T.; Ali, S.; Dar, A. A.; Lal, M. Tetrahedron Lett. 2011, 52, 5157.
doi: 10.1016/j.tetlet.2011.07.113 |
| [8] |
Saidi, M. R.; Azizi, N.; Akbari, E.; Ebrahimi, F. J. Mol. Catal. A: Chem. 2008, 292, 44.
doi: 10.1016/j.molcata.2008.06.003 |
| [9] |
Quadrado, R. F. N.; Macagnan, K. L.; Moreira, A. S.; Rajardo, A. R. Int. J. Biol. Macromol. 2021, 193, 1032.
doi: 10.1016/j.ijbiomac.2021.11.075 pmid: 34800516 |
| [10] |
Kshiar, B.; Shangpliang, Q. R.; Myrboh, B. Synth. Commun. 2018, 48, 1816.
doi: 10.1080/00397911.2018.1468467 |
| [11] |
Xu, S. B.; Li, C. J.; Jia, X. S.; Li, J. J. Org. Chem. 2014, 79, 11161.
doi: 10.1021/jo502209f |
| [12] |
Yang, H.; Ning, Z.; Wang, S.; Li, J.; Wang, Z.; Wang, W. L.; Xu, X. M. Tetrahedron Lett. 2021, 74, 153174.
doi: 10.1016/j.tetlet.2021.153174 |
| [13] |
Karmakar, B.; Banerji, J. Tetrahedron Lett. 2011, 52, 6584.
doi: 10.1016/j.tetlet.2011.09.131 |
| [14] |
Azizi, N.; Khajeh, M.; Hasani, M.; Dezfooli, S. Tetrahedron Lett. 2013, 54, 5407.
|
| [15] |
Ying, A.; Li, Z.; Yang, J.; Liu, S.; Xu, S.; Yan, H.; Wu, C. J. Org. Chem. 2014, 79, 6510.
doi: 10.1021/jo500937a |
| [16] |
Gupta, R.; Yadav, M.; Gaur, R.; Arora, G.; Rana, P.; Yadav, P.; Adholeya, A.; Sharma, R. K. ACS Omega 2019, 4, 21529.
doi: 10.1021/acsomega.9b03237 |
| [17] |
Gawande, M. B.; Branco, P. S.; Varma, R. S. Chem. Soc. Rev. 2013, 42, 3371.
doi: 10.1039/c3cs35480f pmid: 23420127 |
| [18] |
Sharma, R. K.; Dutta, S.; Sharma, S.; Zboril, R.; Varma, R. S.; Gawande, M. B. Green Chem. 2016, 18, 3184.
doi: 10.1039/C6GC00864J |
| [19] |
Saxena, M.; Saxena, R. Mater. Chem. Phys. 2022, 276, 125437.
doi: 10.1016/j.matchemphys.2021.125437 |
| [20] |
Haqjow, H.; Raoufi, F. Res. Chem. Intermed. 2021, 47, 4113.
doi: 10.1007/s11164-021-04522-7 |
| [21] |
Ying, A.; Liu, S.; Li, Z.; Chen, G.; Yang, J.; Yan, H.; Xu, S. Adv. Synth. Catal. 2016, 358, 2116.
doi: 10.1002/adsc.201600145 |
| [22] |
Wang, A.; Sudarsanam, P.; Xu, Y.; Zhang, H.; Li, H.; Yang, S. Green Chem. 2020, 22, 2977.
doi: 10.1039/D0GC00924E |
| [23] |
Lu, X.; Li, S.; Wang, L.; Huang, S.; Liu, Z.; Liu, Y.; Ying, A. Fuel 2022, 310, 122318.
doi: 10.1016/j.fuel.2021.122318 |
| [24] |
Chen, Z.; Yao, J.; Ma, B.; Liu, B.; Kim, J.; Li, H.; Zhu, X.; Zhao, C.; Amde, M. Chemosphere 2022, 291, 132727.
doi: 10.1016/j.chemosphere.2021.132727 |
| [25] |
Ying, Q.; Chen, H.; Shao, P.; Zhou, X.; He, X.; Ye, J.; Zhang, S.; Chen, J.; Wang, L. J. CO2 Util. 2021, 49, 101565.
|
| [26] |
Gawande, M. B.; Branco, P. S.; Varma, R. S. Chem. Soc. Rev. 2013, 42, 3371.
doi: 10.1039/c3cs35480f pmid: 23420127 |
| [27] |
McGuire, G. E.; Schweitzer, G. K.; Thomas, T. A. Inorg. Chem. 1973, 12, 2450.
doi: 10.1021/ic50128a045 |
| [28] |
Deng, R. J.; You, K. Y.; Yi, L.; Zhao, F. F.; Jian, J.; Chen, Z. P.; Liu, P.; Ai, Q. H.; Luo, H. A. Ind. Eng. Chem. Res. 2018, 57, 12993.
doi: 10.1021/acs.iecr.8b02786 |
| [29] |
Azizi, N.; Aryanasab, F.; Torkiyan, L.; Ziyaei, A.; Saidi, M. R. J. Org. Chem. 2006, 71, 3634.
doi: 10.1021/jo060048g |
| [1] | Jing Huang, Yihua Yang, Zhanhui Zhang, Shouxin Liu. Recent Progress on Green Methods and Technologies for Efficient Formation of Amide Bonds [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 409-420. |
| [2] | Yixin Jiang, Boxiao Tang, Haibo Mao, Xuexia Chen, Yangjie Yu, Cuiying Quan, Zhaoyang Xu, Jinhui Shi, Yilin Liu. A Green, Recyclable and Carrier-Free Study for the Coupling Reaction of Alkenes with Aryl Iodides in H2O-Polyethylene Glycol (PEG-200) [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3210-3215. |
| [3] | Dandan Sui, Nannan Cen, Ruoqu Gong, Yang Chen, Wenbo Chen. Supporting-Electrolyte-Free Electrochemical Synthesis of Trifluoromethylated Oxindoles in Continuous Flow [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3239-3245. |
| [4] | Kai Lu, Haoqi Qu, Xi Chen, Hui Qiu, Jing Zheng, Mengtao Ma. Catalyst-Free and Solvent-Free Hydroboration of Alkynes and Alkenes with Catecholborane [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2197-2205. |
| [5] | Baichuan Mo, Chunxia Chen, Jinsong Peng. Research Progress in Application of Lignin and Its Derivatives Supported Metal Catalysts in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1215-1240. |
| [6] | Rui Wang, Lang Gao, Cen Zhou, Xiao Zhang. Haloperfluoroalkylation of Unactivated Terminal Alkenes over Phenylphenothiazine-Based Porous Organic Polymers [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1136-1145. |
| [7] | Qiyang Li, Haiyan Zhang, Wenbo Liu. Research Progress in Transition-Metal-Free C—Si Bond Formation [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3470-3490. |
| [8] | Shiwei Yu, Zhaohua Chen, Qi Chen, Shuting Lin, Jinping He, Guanshen Tao, Zhaoyang Wang. Research Progress in Synthesis and Application of Thiosulfonates [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2322-2330. |
| [9] | Qingyun Gu, Zhenfeng Cheng, Xiaobao Zeng. Electrochemical Oxidative Trifluoromethylation of α-Oxoketene Ketene Dithioacetals with CF3SO2Na [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1537-1544. |
| [10] | Yu Zheng, Shencheng Qian, Pengcheng Xu, Binnan Zheng, Shenlin Huang. Electrochemical Oxidative Thiocyanosulfonylation of Aryl Acetylenes [J]. Chinese Journal of Organic Chemistry, 2022, 42(12): 4275-4281. |
| [11] | Hongxia Li, Peng Chen, Zhilin Wu, Yuhan Lu, Junmei Peng, Jingyang Chen, Weimin He. Electrochemical Oxidative Cross-Dehydrogenative Coupling of Five-Membered Aromatic Heterocycles with NH4SCN [J]. Chinese Journal of Organic Chemistry, 2022, 42(10): 3398-3404. |
| [12] | Yaoyao Zhang, Lijie Zhou, Biao Han, Weishuang Li, Bojie Li, Lei Zhu. Research Progress of Chitosan Supported Copper Catalyst in Organic Reactions [J]. Chinese Journal of Organic Chemistry, 2022, 42(1): 33-53. |
| [13] | Zeyin Meng, Chengtao Feng, Kun Xu. Recent Advances in the Electrochemical Formation of Carbon-Nitrogen Bonds [J]. Chinese Journal of Organic Chemistry, 2021, 41(7): 2535-2570. |
| [14] | Zhiheng Zhao, Ming Li, Yaqin Zhou, Yonghui He, Lizhu Zhang, Ganpeng Li, Lijun Gu. Synthesis of 1,2,4-Triazoles via the Electrochemical Oxidative [3+2] Annulation [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2476-2484. |
| [15] | Mei Wu, Ling Yu, Huiqing Hou, Houzheng Chen, Qinglong Zhuang, Sunying Zhou, Xiaoyan Lin. Electrochemistry-Enabled Copper-Catalyzed Oxidation of Benzyl Alcohols for the Preparation of Quinazolinones in Water [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2326-2334. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||