Chinese Journal of Organic Chemistry ›› 2023, Vol. 43 ›› Issue (2): 686-696.DOI: 10.6023/cjoc202206030 Previous Articles Next Articles
李猛†, 夏东国†, 王云霄, 程祥, 巩杰秀, 陈耀, 吕献海*( )
)
收稿日期:2022-06-18
修回日期:2022-09-04
发布日期:2022-10-24
作者简介:基金资助:
Meng Li†, Dongguo Xia†, Yunxiao Wang, Xiang Cheng, Jiexiu Gong, Yao Chen, Xianhai Lü( )
)
Received:2022-06-18
Revised:2022-09-04
Published:2022-10-24
Contact:
*E-mail: About author:Supported by:Share
Meng Li, Dongguo Xia, Yunxiao Wang, Xiang Cheng, Jiexiu Gong, Yao Chen, Xianhai Lü. Design, Synthesis and Antifungal Bioactivity Evaluation of Thiazole Benzoate Derivatives[J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 686-696.
| Compd. | R1 | R2 | Inhibition ratea/% | ||||
|---|---|---|---|---|---|---|---|
| B. cinereab | V. malic | S. sclerotiorumd | |||||
| 1 | Phenyl | H | 15.4±0.7 | 25.8±1.2 | 30.2±1.3 | ||
| 2 | 1-Naphthyl | H | 45.6±0.9 | 16.9±1.4 | 10.5±1.8 | ||
| 3 | Isopropyl | H | 72.3±1.5 | 72.6±0.9 | 83.2±1.1 | ||
| 4 | 2-Cl-Phenyl | H | 22.6±1.5 | 41.3±0.8 | 20.1±0.8 | ||
| 5 | 3-Cl-Phenyl | H | 77.4±2.2 | 38.7±1.6 | 56.0±1.4 | ||
| 6 | 4-Cl-Phenyl | H | 32.3±1.1 | 12.9±0.7 | 26.3±2.9 | ||
| 7 | 2-CH3-Phenyl | H | 13.1±2.4 | 21.6±1.2 | 43.5±0.9 | ||
| 8 | 3-CH3-Phenyl | H | 95.3±3.5 | 61.2±1.8 | 94.9±1.6 | ||
| 9 | 4-CH3-Phenyl | H | 23.0±1.5 | 31.5±1.4 | 18.4±0.7 | ||
| 10 | Isopropyl | 2-Cl | 30.5±0.9 | 61.5±0.3 | 20.1±0.3 | ||
| 11 | Isopropyl | 3-Cl | 86.9±2.0 | 53.4±2.5 | 90.9±1.1 | ||
| 12 | Isopropyl | 4-Cl | 83.3±1.3 | 41.9±2.2 | 85.2±0.3 | ||
| 13 | Isopropyl | 2-CH3 | 16.4±0.5 | 63.3±1.0 | 67.6±1.4 | ||
| 14 | Isopropyl | 3-CH3 | 90.6±0.7 | 81.6±2.4 | 88.2±2.1 | ||
| 15 | Isopropyl | 4-CH3 | 44.3±1.9 | 65.3±2.0 | 65.2±1.6 | ||
| 16 | Isopropyl | 2-CF3 | 19.1±0.6 | 61.2±1.3 | 63.8±0.3 | ||
| 17 | Isopropyl | 3-CF3 | 48.3±1.3 | 61.6±0.5 | 33.6±0.6 | ||
| 18 | Isopropyl | 4-CF3 | 30.6±1.7 | 57.5±1.1 | 65.0±1.2 | ||
| 19 | 3-Cl-Phenyl | 2-Cl | 82.2±0.6 | 55.1±1.9 | 56.0±1.8 | ||
| 20 | 3-Cl-Phenyl | 3-Cl | 11.5±0.5 | 30.6±0.7 | 5.1±1.2 | ||
| 21 | 3-Cl-phenyl | 4-Cl | 82.9±2.0 | 51.0±1.5 | 85.3±0.9 | ||
| 22 | 3-Cl-Phenyl | 2-CH3 | 88.2±1.5 | 12.3±0.3 | 95.1±1.4 | ||
| 23 | 3-Cl-Phenyl | 3-CH3 | 16.4±1.9 | 9.8±1.3 | 61.6±0.4 | ||
| 24 | 3-Cl-Phenyl | 4-CH3 | 40.1±0.8 | 8.2±1.4 | 81.6±0.2 | ||
| 25 | 3-Cl-Phenyl | 2-CF3 | 45.3±9.5 | 14.5±0.2 | 56.2±1.3 | ||
| 26 | 3-Cl-Phenyl | 3-CF3 | 35.8±1.2 | 16.2±0.6 | 40.8±1.8 | ||
| 27 | 3-Cl-Phenyl | 4-CF3 | 21.5±0.4 | 65.1±2.2 | 30.8±1.6 | ||
| 28 | 3-CH3-Phenyl | 2-Cl | 84.4±0.6 | 55.6±1.2 | 26.4±1.3 | ||
| 30 | 3-CH3-Phenyl | 4-Cl | 60.9±1.2 | 36.9±0.5 | 40.6±0.7 | ||
| 31 | 3-CH3-Phenyl | 2-CH3 | 57.3±2.0 | 45.4±1.3 | 35.1±0.3 | ||
| 32 | 3-CH3-Phenyl | 3-CH3 | 56.7±0.7 | 26.4±0.5 | 16.7±1.4 | ||
| Compd. | R1 | R2 | Inhibition ratea/% | ||||
| B. cinereab | V. malic | S. sclerotiorumd | |||||
| 33 | 3-CH3-Phenyl | 4-CH3 | 44.3±0.5 | 61.0±2.2 | 19.2±2.1 | ||
| 34 | 3-CH3-Phenyl | 2-CF3 | 58.0±1.5 | 20.3±0.4 | 15.6±1.5 | ||
| 35 | 3-CH3-Phenyl | 3-CF3 | 65.4±1.9 | 9.1±1.5 | 6.4±0.5 | ||
| 36 | 3-CH3-Phenyl | 4-CF3 | 45.6±1.3 | 30.7±1.1 | 46.5±1.8 | ||
| Boscalid | - | - | 100.0 | 95.3 | 100.0 | ||
| Compd. | R1 | R2 | Inhibition ratea/% | ||||
|---|---|---|---|---|---|---|---|
| B. cinereab | V. malic | S. sclerotiorumd | |||||
| 1 | Phenyl | H | 15.4±0.7 | 25.8±1.2 | 30.2±1.3 | ||
| 2 | 1-Naphthyl | H | 45.6±0.9 | 16.9±1.4 | 10.5±1.8 | ||
| 3 | Isopropyl | H | 72.3±1.5 | 72.6±0.9 | 83.2±1.1 | ||
| 4 | 2-Cl-Phenyl | H | 22.6±1.5 | 41.3±0.8 | 20.1±0.8 | ||
| 5 | 3-Cl-Phenyl | H | 77.4±2.2 | 38.7±1.6 | 56.0±1.4 | ||
| 6 | 4-Cl-Phenyl | H | 32.3±1.1 | 12.9±0.7 | 26.3±2.9 | ||
| 7 | 2-CH3-Phenyl | H | 13.1±2.4 | 21.6±1.2 | 43.5±0.9 | ||
| 8 | 3-CH3-Phenyl | H | 95.3±3.5 | 61.2±1.8 | 94.9±1.6 | ||
| 9 | 4-CH3-Phenyl | H | 23.0±1.5 | 31.5±1.4 | 18.4±0.7 | ||
| 10 | Isopropyl | 2-Cl | 30.5±0.9 | 61.5±0.3 | 20.1±0.3 | ||
| 11 | Isopropyl | 3-Cl | 86.9±2.0 | 53.4±2.5 | 90.9±1.1 | ||
| 12 | Isopropyl | 4-Cl | 83.3±1.3 | 41.9±2.2 | 85.2±0.3 | ||
| 13 | Isopropyl | 2-CH3 | 16.4±0.5 | 63.3±1.0 | 67.6±1.4 | ||
| 14 | Isopropyl | 3-CH3 | 90.6±0.7 | 81.6±2.4 | 88.2±2.1 | ||
| 15 | Isopropyl | 4-CH3 | 44.3±1.9 | 65.3±2.0 | 65.2±1.6 | ||
| 16 | Isopropyl | 2-CF3 | 19.1±0.6 | 61.2±1.3 | 63.8±0.3 | ||
| 17 | Isopropyl | 3-CF3 | 48.3±1.3 | 61.6±0.5 | 33.6±0.6 | ||
| 18 | Isopropyl | 4-CF3 | 30.6±1.7 | 57.5±1.1 | 65.0±1.2 | ||
| 19 | 3-Cl-Phenyl | 2-Cl | 82.2±0.6 | 55.1±1.9 | 56.0±1.8 | ||
| 20 | 3-Cl-Phenyl | 3-Cl | 11.5±0.5 | 30.6±0.7 | 5.1±1.2 | ||
| 21 | 3-Cl-phenyl | 4-Cl | 82.9±2.0 | 51.0±1.5 | 85.3±0.9 | ||
| 22 | 3-Cl-Phenyl | 2-CH3 | 88.2±1.5 | 12.3±0.3 | 95.1±1.4 | ||
| 23 | 3-Cl-Phenyl | 3-CH3 | 16.4±1.9 | 9.8±1.3 | 61.6±0.4 | ||
| 24 | 3-Cl-Phenyl | 4-CH3 | 40.1±0.8 | 8.2±1.4 | 81.6±0.2 | ||
| 25 | 3-Cl-Phenyl | 2-CF3 | 45.3±9.5 | 14.5±0.2 | 56.2±1.3 | ||
| 26 | 3-Cl-Phenyl | 3-CF3 | 35.8±1.2 | 16.2±0.6 | 40.8±1.8 | ||
| 27 | 3-Cl-Phenyl | 4-CF3 | 21.5±0.4 | 65.1±2.2 | 30.8±1.6 | ||
| 28 | 3-CH3-Phenyl | 2-Cl | 84.4±0.6 | 55.6±1.2 | 26.4±1.3 | ||
| 30 | 3-CH3-Phenyl | 4-Cl | 60.9±1.2 | 36.9±0.5 | 40.6±0.7 | ||
| 31 | 3-CH3-Phenyl | 2-CH3 | 57.3±2.0 | 45.4±1.3 | 35.1±0.3 | ||
| 32 | 3-CH3-Phenyl | 3-CH3 | 56.7±0.7 | 26.4±0.5 | 16.7±1.4 | ||
| Compd. | R1 | R2 | Inhibition ratea/% | ||||
| B. cinereab | V. malic | S. sclerotiorumd | |||||
| 33 | 3-CH3-Phenyl | 4-CH3 | 44.3±0.5 | 61.0±2.2 | 19.2±2.1 | ||
| 34 | 3-CH3-Phenyl | 2-CF3 | 58.0±1.5 | 20.3±0.4 | 15.6±1.5 | ||
| 35 | 3-CH3-Phenyl | 3-CF3 | 65.4±1.9 | 9.1±1.5 | 6.4±0.5 | ||
| 36 | 3-CH3-Phenyl | 4-CF3 | 45.6±1.3 | 30.7±1.1 | 46.5±1.8 | ||
| Boscalid | - | - | 100.0 | 95.3 | 100.0 | ||
| Fungi | Compd. | EC50/(mg•L-1) | 95% confidence interval | Regression equationa | Rb |
|---|---|---|---|---|---|
| B. cinerea | 3 | 32.61 | 29.13~36.99 | y=5.581x-8.446 | 0.998 |
| 5 | 31.60 | 28.24~35.81 | y=5.550x-8.323 | 0.988 | |
| 8 | 13.82 | 12.27~15.58 | y=4.690x-5.349 | 0.989 | |
| 11 | 21.17 | 18.97~23.72 | y=5.422x-7.188 | 0.989 | |
| 12 | 21.81 | 14.06~40.87 | y=3.120x-4.176 | 0.942 | |
| 14 | 15.31 | 13.56~17.32 | y=4.513x-5.347 | 0.994 | |
| 19 | 21.85 | 19.01~25.43 | y=3.941x-5.010 | 0.986 | |
| 21 | 23.36 | 15.85~40.05 | y=3.498x-4.787 | 0.962 | |
| 22 | 19.25 | 17.11~21.77 | y=4.765x-6.120 | 0.995 | |
| 28 | 23.17 | 20.27~26.85 | y=3.974x-5.420 | 0.980 | |
| Boscalid | 0.88 | 0.03~1.77 | y=2.340x+0.134 | 0.971 | |
| V. mali | 3 | 30.74 | 27.42~34.90 | y=5.375x-7.996 | 0.999 |
| 14 | 15.90 | 13.82~18.40 | y=3.576x-4.296 | 0.980 | |
| Boscalid | 13.44 | 11.55~16.13 | y=3.804x-4.292 | 0.978 | |
| S. sclerotiorum | 3 | 12.07 | 10.22~14.20 | y=2.919x-3.156 | 0.988 |
| 8 | 3.66 | 2.57~4.70 | y=2.370x-1.335 | 0.986 | |
| 11 | 8.79 | 7.28~10.46 | y=2.689x-2.538 | 0.988 | |
| 12 | 15.74 | 13.76~18.08 | y=3.830x-4.584 | 0.986 | |
| 14 | 12.38 | 10.80~14.20 | y=3.755x-4.104 | 0.986 | |
| 21 | 22.90 | 20.41~25.89 | y=5.008x-6.810 | 0.993 | |
| 22 | 13.81 | 9.92~19.38 | y=5.166x-5.891 | 0.983 | |
| 24 | 16.59 | 14.38~19.27 | y=3.492x-4.260 | 0.985 | |
| Boscalid | 0.95 | 0.53~1.35 | y=2.052x+0.049 | 0.960 |
| Fungi | Compd. | EC50/(mg•L-1) | 95% confidence interval | Regression equationa | Rb |
|---|---|---|---|---|---|
| B. cinerea | 3 | 32.61 | 29.13~36.99 | y=5.581x-8.446 | 0.998 |
| 5 | 31.60 | 28.24~35.81 | y=5.550x-8.323 | 0.988 | |
| 8 | 13.82 | 12.27~15.58 | y=4.690x-5.349 | 0.989 | |
| 11 | 21.17 | 18.97~23.72 | y=5.422x-7.188 | 0.989 | |
| 12 | 21.81 | 14.06~40.87 | y=3.120x-4.176 | 0.942 | |
| 14 | 15.31 | 13.56~17.32 | y=4.513x-5.347 | 0.994 | |
| 19 | 21.85 | 19.01~25.43 | y=3.941x-5.010 | 0.986 | |
| 21 | 23.36 | 15.85~40.05 | y=3.498x-4.787 | 0.962 | |
| 22 | 19.25 | 17.11~21.77 | y=4.765x-6.120 | 0.995 | |
| 28 | 23.17 | 20.27~26.85 | y=3.974x-5.420 | 0.980 | |
| Boscalid | 0.88 | 0.03~1.77 | y=2.340x+0.134 | 0.971 | |
| V. mali | 3 | 30.74 | 27.42~34.90 | y=5.375x-7.996 | 0.999 |
| 14 | 15.90 | 13.82~18.40 | y=3.576x-4.296 | 0.980 | |
| Boscalid | 13.44 | 11.55~16.13 | y=3.804x-4.292 | 0.978 | |
| S. sclerotiorum | 3 | 12.07 | 10.22~14.20 | y=2.919x-3.156 | 0.988 |
| 8 | 3.66 | 2.57~4.70 | y=2.370x-1.335 | 0.986 | |
| 11 | 8.79 | 7.28~10.46 | y=2.689x-2.538 | 0.988 | |
| 12 | 15.74 | 13.76~18.08 | y=3.830x-4.584 | 0.986 | |
| 14 | 12.38 | 10.80~14.20 | y=3.755x-4.104 | 0.986 | |
| 21 | 22.90 | 20.41~25.89 | y=5.008x-6.810 | 0.993 | |
| 22 | 13.81 | 9.92~19.38 | y=5.166x-5.891 | 0.983 | |
| 24 | 16.59 | 14.38~19.27 | y=3.492x-4.260 | 0.985 | |
| Boscalid | 0.95 | 0.53~1.35 | y=2.052x+0.049 | 0.960 |

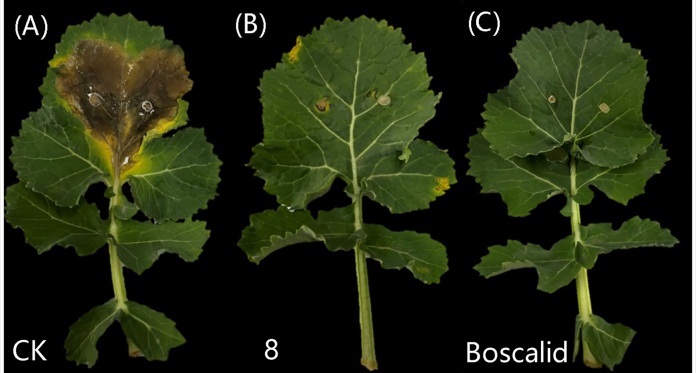
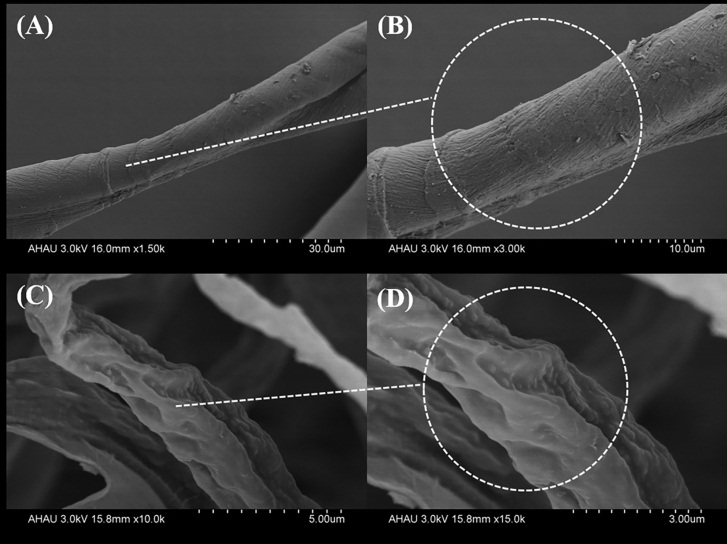
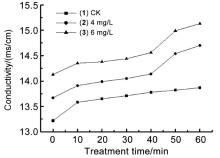
| [1] |
Bolton, M. D.; Thomma, B. P. H. J.; Nelson, B. D. Mol. Plant. Pathol. 2006, 7. 1.
|
| [2] |
Huang, X.-P.; Song, Y.-F.; Li, B.-X.; Mu, W.; Liu, F. Crop Prot. 2019, 122, 42.
doi: 10.1016/j.cropro.2019.04.010 |
| [3] |
Zhu, J.-K.; Gao, J-M.; Yang, C.-J.; Shang, X.-F.; Zhao, Z.-M.; Lawoe, R.-K.; Zhou, R.; Sun, Y.; Yin, X.-D.; Liu, Y. Q. J. Agric. Food Chem. 2020, 68, 2306.
doi: 10.1021/acs.jafc.9b06793 |
| [4] |
Cheng, X.; Wang, W.; Wang, Y.-X.; Xia, D.-G.; Yin, F.; Liu, Q.; Luo, H.; Li, M.; Zhang, C.-Q.; Cao, H.-Q.; Lv, X.-H. J. Agric. Food Chem. 2021, 69, 11395.
doi: 10.1021/acs.jafc.1c02454 |
| [5] |
Yao, T.-T.; Fang, S.-W.; Li, Z.-S.; Xiao, D.-X.; Cheng, J.-L.; Ying, H.-Z.; Du, Y.-J.; Zhao, J.-H.; Dong, X.-W. J. Agric. Food Chem. 2017, 65, 3204.
doi: 10.1021/acs.jafc.7b00249 |
| [6] |
Wang, W.; Zhang, S.; Wang, J.-H.; Wu, F.-R.; Wang, T.; Xu, G. J. Agric. Food Chem. 2021, 69, 491.
doi: 10.1021/acs.jafc.0c06700 |
| [7] |
Wang, X.; Wang, A.; Qiu, L.; Chen, M.; Lu, A.; Li, G.; Yang, C.; Xue, W. J. Agric. Food Chem. 2020, 68, 14426.
doi: 10.1021/acs.jafc.0c03736 |
| [8] |
Wu, Z.; Park, H.-Y.; Xie, D.; Yang, J.; Hou, S.; Shahzad, N.; Kim, C. K.; Yang, S. J. Agric. Food Chem. 2021, 69, 1214.
doi: 10.1021/acs.jafc.0c05702 |
| [9] |
Guo, X.; Zhao, B.; Fan, Z.; Yang, D.-Y.; Belskaya, N. P. J. Agric. Food Chem. 2019, 67, 1647.
doi: 10.1021/acs.jafc.8b06935 |
| [10] |
Chen, L.; Zhao, Bin.; Fan, Z.-J.; Hu, M.-X.; Li, Q.; Hu, W.-H.; Li, J.-W.; Zhang, J.-L. J. Agric. Food Chem. 2019, 67, 12357.
doi: 10.1021/acs.jafc.9b03891 |
| [11] |
Lu, Q.; Yu, Q.; Zhu, Y.-B.; Weng, J.-Q.; Yuan, J.; Hu, D.-X.; Chen, J.; Liu, X.-H.; Tan, C.-X. J. Mol. Struct. 2019, 1180, 780.
doi: 10.1016/j.molstruc.2018.12.068 |
| [12] |
Wu, Q.; Zhao, B.; Fan, Z.-J.; Guo, X.-F.; Yang, D.-Y.; Zang, N.-L.; Yu, B.; Zhou, S.; Zhao, J.-B.; Chen, F. J. Agric. Food Chem. 2019, 67, 1360.
doi: 10.1021/acs.jafc.8b06054 |
| [13] |
Yu, B.; Zhou, S.; Cao, L.-X.; Hao, Z.-S.; Yang, D.-Y.; Guo, X.-F.; Zhang, N.-L.; Bakulev, V.A.; Fan, Z.-J. J. Agric. Food Chem. 2020, 68, 7093.
doi: 10.1021/acs.jafc.0c00062 |
| [14] |
Jr, H; Phares, H.F. Otto, E.J.J.o.I.D. J. Invest. Dermatol. 1964, 42, 479.
doi: 10.1038/jid.1964.101 |
| [15] |
Yang, Y.; Dong, F.-S.; Liu, X.-G.; Xu, J.; Wu, X.-H.; Zheng, Y.-Q. Environ. Pollut. 2020, 265, 115031.
doi: 10.1016/j.envpol.2020.115031 |
| [16] |
Kim, D. S.; Chun, S. J.; Jeon, J. J.; Lee, S. W.; Joe, G. H. Pest. Manage. Sci. 2004, 60, 1007.
doi: 10.1002/ps.873 |
| [17] |
Cohen, Y. J.; Rubin, A. E.; Galperin, M. Phytoparasitica 2018, 46, 689.
doi: 10.1007/s12600-018-0702-6 |
| [18] |
Gao, Y.-Y.; Zhao, X.-J.; Sun, X.-F.; Wang, Z.; Zhang, J.; Li, L.-S.; Shi, H.-Y.; Wang, M.-H. J. Agric. Food Chem. 2021, 69, 3289.
doi: 10.1021/acs.jafc.0c04163 |
| [19] |
Siegenthaler, T. B.; Hansen, Z. Plant Dis. 2021, 105, 1943.
|
| [20] |
Guo, Y.; Fan, J.-P.; Zhang, Q.; Bao, C.-N.; Liu, Z.-Y.; Yang, R. G. Bioorg. Med. Chem. Lett. 2019, 29, 179.
doi: 10.1016/j.bmcl.2018.12.002 |
| [21] |
Soliman, N. N.; Salam, M. A. E.; Fadda, A. A.; Abdel-Motaal, M. J. Agric. Food Chem. 2020, 68,5790.
|
| [22] |
Liu, H.; Xia, D.-G.; Chu, Z.-W.; Hu, R.; Lv, X.-H. Bioorg. Chem. 2020, 100, 103907.
doi: 10.1016/j.bioorg.2020.103907 |
| [23] |
Mattson, A. E.; Bharadwaj, A. R.; Zuhl, A. M.; Scheidt, K. A. J. Org. Chem. 2006, 71. 5715.
|
| [24] |
Mattson, A. E.; Zuhl, A. M.; Reynolds, T. E.; Scheidt, K. A. J. Am. Chem. Soc. 2006, 128, 4932.
pmid: 16608309 |
| [25] |
Ren, Z.-L.; Liu, H.; Jiao, D.; Hu, H.-T.; Wang, Wei.; Gong, J.-X.; Wang, A.-L.; Cao, H.-Q.; Lv, X.-H. Drug Dev. Res. 2018, 79, 307.
doi: 10.1002/ddr.21469 |
| [26] |
Liu, H.; Xia, D.-G.; Hu, R.; Wang, W.; Cheng, X.; Wang, A.-L.; Zhang, Q.; Lv, X.-H. Pestic. Biochem. Phys. 2020, 163, 271.
doi: 10.1016/j.pestbp.2019.11.024 |
| [27] |
Wang, W.; Cheng, X.; Cui, X.; Xia, D.-G.; Wang, Z.-Q.; Lv, X.-H. Pest. Manage. Sci. 2021, 77, 3529.
doi: 10.1002/ps.6406 |
| [28] |
Yin, X.-D.; Ma, K.-Y.; Wang, Y.-L.; Sun, Y.; Shang, X.-F.; Zhao, Z.-M.; Wang, R.-X; Chen, Y.-J.; Zhu, J.-K.; Liu, Y.-Q. J. Agric. Food Chem. 2020, 68, 11096.
doi: 10.1021/acs.jafc.0c01322 |
| [29] |
Magdalena, E.-S.; Agnieszka, N.; Agata, C.; Monika, Ś.; Anna, O.; Dorota, Ż. Food Chem. 2021, 350, 129218.
doi: 10.1016/j.foodchem.2021.129218 |
| [30] |
Sabatino, V.; Rebelein, J. G.; Ward, T. R. J. Am. Chem. Soc. 2019, 141, 17048.
doi: 10.1021/jacs.9b07193 pmid: 31503474 |
| [1] | Xiaoying Jia, Jiaxia Pu, Lirong Han, Qinghan Li. Research Progress in the Synthesis of Benzo[d]pentamembered Heterocyclic Thioethers Containing Two Heteroatoms [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 18-40. |
| [2] | Feng Wang, Yu Chen, Hongyan Pei, Jing Zhang, Lixin Zhang. Design, Synthesis and Antifungal Activities of Novel 1,2,4-Oxadiazole Derivatives Containing Piperidine [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2826-2836. |
| [3] | Meng Liu, Yanru Huang, Xiaofei Sun, Lijun Tang. An “Aggregation-Induced Emission+Excited-State Intramolecular Proton Transfer” Mechanisms-Based Benzothiazole Derived Fluorescent Probe and Its ClO– Recognition [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 345-351. |
| [4] | Wei Chen, Simin Lei, Yuxin Lan, Haojian Xu, Pingbin Yu, Rui Zhang, Run Wu, Yang Chen. Design, Synthesis and Antifungal Activities of Novel Quinazolinone Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2164-2171. |
| [5] | Xiu Wang, Wengui Duan, Guishan Lin, Baoyu Li, Wenjing Zhang, Fuhou Lei. Synthesis, Antifungal Activity, Three-Dimensional Quantitative Structure-Activity Relationship and Molecular Docking Study of 4-Acyl-3-amino-1,2,4-triazole-thioether Derivatives Containing Natural Pinene Structure [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 871-883. |
| [6] | Wei Wang, Furan Wu, Yidan Ma, Dan Xu, Gong Xu. Study on Synthesis and Antifungal Activity of Novel Benzamides Containing Substituted Pyrazole Unit [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 607-618. |
| [7] | Yucheng Cui, Meihua Chen, Guishan Lin, Wengui Duan, Qingmin Li, Renxuan Zou, Bo Cen. Synthesis, Antifungal Activity and Molecular Docking Study of 1,3,4-Thiadiazole-Urea Compounds Containing gem-Dimethylcyclopropane Ring Structure [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3784-3797. |
| [8] | Honglin Dai, Xiaojie Si, Lingling Chi, Hao Wang, Chao Gao, Zhengjie Wang, Limin Liu, Jiajie Ma, Fuqiang Yu, Hongmin Liu, Yu Ke, Qiurong Zhang. Synthesis and Antitumor Activity Evaluation of 2,4,6-Trisubstituted Quinazoline Derivatives Containing Thiazole Structure [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3853-3862. |
| [9] | Shu Chen, Yingying Shao, Xinhao Fu, Qingwu Chen, Xiaohua Du, Chengxia Tan. Design, Synthesis and Insecticidal Activities of Pyridyl Thiazole Diamide Compounds [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3870-3879. |
| [10] | Qinjiao Fu, Ruiqin Zhang, Huanyi Qiu, Renchao Ma, Yongmin Ma. A New Method for the Synthesis of 2-Arylbenzothiazoles Oxidized by Selectfluor [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3585-3592. |
| [11] | Qi Lu, Feixia Ye, Xiaotong Sun, Jianquan Weng, Qian Yu, Dexuan Hu. Design and Synthesis of Novel Nature-Inspired Stilbene Analogues as Potential Topoisomerase 1 Inhibitors [J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3321-3329. |
| [12] | Tao Zhang, Nianxian Li, Nanqian Zhou, Wen Ma, Haiyuan Wei, Bingxin Zhang, Lianghui Chen, Guangfan Hai, Yingchao Duan, Suping Bai. Design, Synthesis and Biological Evaluation of Novel Thiazole-Fused Glaucocalyxin A Derivatives [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2393-2400. |
| [13] | Weiwei Wang, Yu Zhao, Xinlei Liu, Jiazhen Jiang, Ming'an Wang. Synthesis and Antifungal Activity of 3-Aryl-7-methyl- 7-hydroxy-2-octen-6-olide [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2343-2353. |
| [14] | Xiaoping Zhang, Guiyong Jin, Zhifei Chen, Qingfu Wang, Sensen Zhao, Zhiyong Wu, Shuai Wan, Gaolei Xi, Xu Zhao. Synthesis and Antioxidant Properties of Pyrazine-Thiazole Bi-heteroaryl Compounds [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2445-2453. |
| [15] | Hongbo Dong, Weiwei Wang, Yu Zhao, Xinlei Liu, Ming'an Wang. Synthesis and Antifungal Activity of 3,7-Dimethyl-7-hydroxy-2-octen-6-olide Analogues [J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1646-1657. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||