Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (8): 3321-3329.DOI: 10.6023/cjoc202102031 Previous Articles Next Articles
NOTES
陆棋a, 叶飞霞a, 孙晓彤a, 翁建全a,*( ), 余茜b,*(
), 余茜b,*( ), 胡德玄c
), 胡德玄c
收稿日期:2021-02-19
修回日期:2021-04-16
发布日期:2021-05-14
通讯作者:
翁建全, 余茜
基金资助:
Qi Lua, Feixia Yea, Xiaotong Suna, Jianquan Wenga( ), Qian Yub(
), Qian Yub( ), Dexuan Huc
), Dexuan Huc
Received:2021-02-19
Revised:2021-04-16
Published:2021-05-14
Contact:
Jianquan Weng, Qian Yu
Supported by:Share
Qi Lu, Feixia Ye, Xiaotong Sun, Jianquan Weng, Qian Yu, Dexuan Hu. Design and Synthesis of Novel Nature-Inspired Stilbene Analogues as Potential Topoisomerase 1 Inhibitors[J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3321-3329.
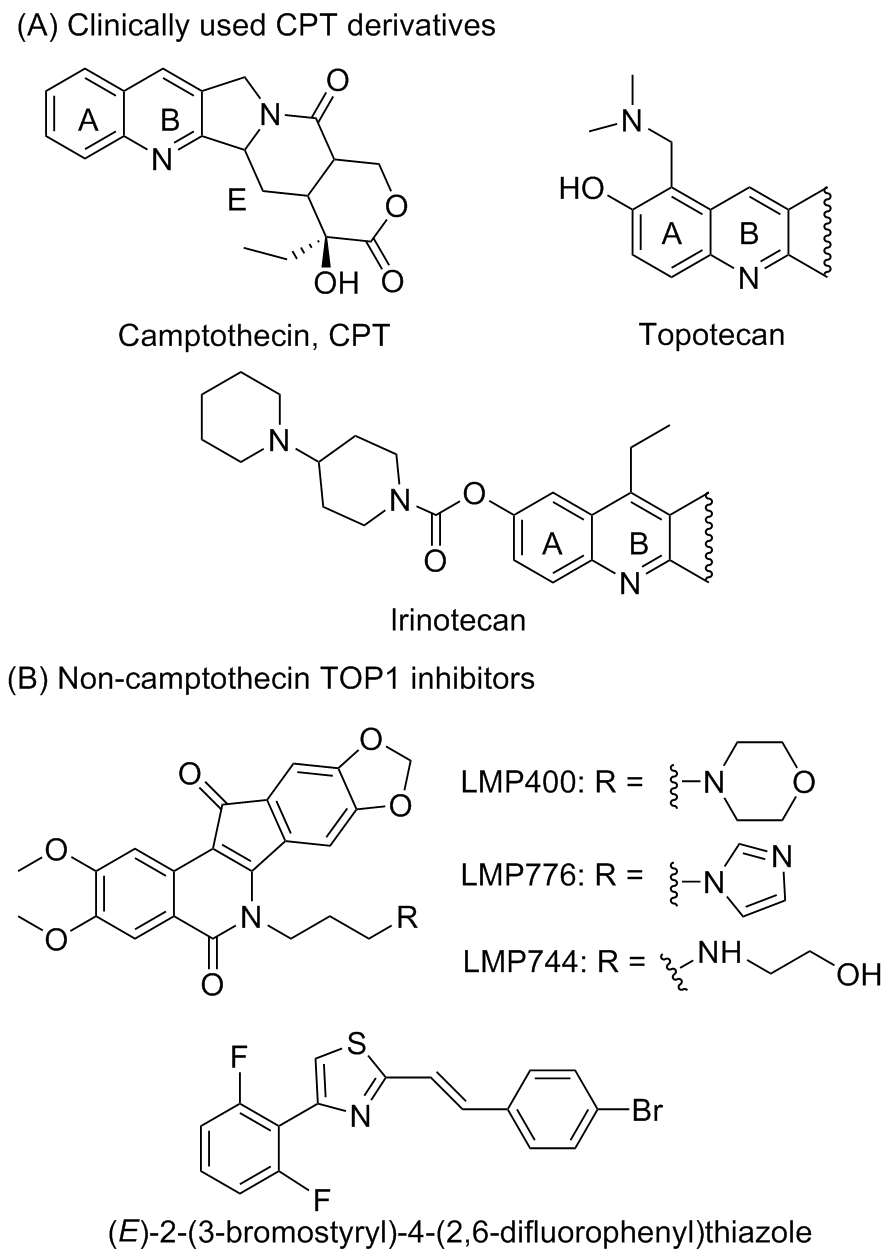
| Compd. | X | R | Relaxation assaya | Compd. | X | R | Relaxation assaya |
|---|---|---|---|---|---|---|---|
| 6a | F | H | + | 6m | F | 2,4-Cl2 | ++ |
| 6b | F | 2-Me | + | 6n | F | 2-Br | ++ |
| 6c | F | 3-Me | + | 6o | F | 3-Br | + |
| 6d | F | 4-Me | + | 6p | Cl | 4-CF3 | ++ |
| 6e | F | 4-CF3 | ++ | 6q | Cl | 4-tert-Bu | + |
| 6f | F | 4-tert-Bu | + | 6r | Cl | 3-OMe | + |
| 6g | F | 2-OMe | + | 6s | Cl | 3,4,5-(OMe)3 | + |
| 6h | F | 3-OMe | + | 6t | Cl | 2-F | + |
| 6i | F | 4-OMe | + | 6u | Cl | 3-Br | + |
| 6j | F | 4-F | ++ | 6v | Br | 2-F | ++ |
| 6k | F | 2-Cl | +++ | 6w | Br | 4-F | ++ |
| 6l | F | 4-Cl | ++ |
| Compd. | X | R | Relaxation assaya | Compd. | X | R | Relaxation assaya |
|---|---|---|---|---|---|---|---|
| 6a | F | H | + | 6m | F | 2,4-Cl2 | ++ |
| 6b | F | 2-Me | + | 6n | F | 2-Br | ++ |
| 6c | F | 3-Me | + | 6o | F | 3-Br | + |
| 6d | F | 4-Me | + | 6p | Cl | 4-CF3 | ++ |
| 6e | F | 4-CF3 | ++ | 6q | Cl | 4-tert-Bu | + |
| 6f | F | 4-tert-Bu | + | 6r | Cl | 3-OMe | + |
| 6g | F | 2-OMe | + | 6s | Cl | 3,4,5-(OMe)3 | + |
| 6h | F | 3-OMe | + | 6t | Cl | 2-F | + |
| 6i | F | 4-OMe | + | 6u | Cl | 3-Br | + |
| 6j | F | 4-F | ++ | 6v | Br | 2-F | ++ |
| 6k | F | 2-Cl | +++ | 6w | Br | 4-F | ++ |
| 6l | F | 4-Cl | ++ |
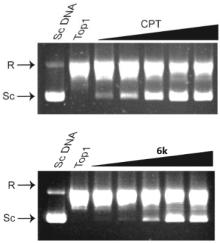


| Compd. | IC50/(µmol•L–1) | Compd. | IC50/(µmol•L–1) | ||
|---|---|---|---|---|---|
| MCF-7 | HCT116 | MCF-7 | HCT116 | ||
| 6a | 22.16±5.01 | 15.78±1.89 | 6n | 20.95±1.41 | 58.38±3.49 |
| 6b | >100 | >100 | 6o | >100 | >100 |
| 6c | >100 | >100 | 6p | >100 | >100 |
| 6d | 95.52±3.11 | 31.87±4.29 | 6q | >100 | >100 |
| 6e | 6.31±0.51 | 0.72±0.09 | 6r | 61.92±8.05 | 5.26±0.35 |
| 6f | 59.42±4.21 | 6.47±0.62 | 6s | >100 | >100 |
| 6g | 49.53±9.08 | 17.8±1.16 | 6t | >100 | >100 |
| 6h | 29.85±5.47 | 4.99±0.80 | 6u | >100 | 62.91±2.34 |
| 6i | 57.20±1.79 | 17.32±1.05 | 6v | 64.57±1.45 | 37.18±6.65 |
| 6j | 49.64±3.58 | 8.06±0.92 | 6w | >100 | 63.25±6.17 |
| 6k | 2.29±1.08 | 7.37±1.15 | vp-16a | 24.68±3.12 | 18.95±1.35 |
| 6l | 13.45±2.46 | 3.07±0.53 | CPTa | 0.34 | 0.012 |
| 6m | 72.43±2.15 | 4.79±0.73 | |||
| Compd. | IC50/(µmol•L–1) | Compd. | IC50/(µmol•L–1) | ||
|---|---|---|---|---|---|
| MCF-7 | HCT116 | MCF-7 | HCT116 | ||
| 6a | 22.16±5.01 | 15.78±1.89 | 6n | 20.95±1.41 | 58.38±3.49 |
| 6b | >100 | >100 | 6o | >100 | >100 |
| 6c | >100 | >100 | 6p | >100 | >100 |
| 6d | 95.52±3.11 | 31.87±4.29 | 6q | >100 | >100 |
| 6e | 6.31±0.51 | 0.72±0.09 | 6r | 61.92±8.05 | 5.26±0.35 |
| 6f | 59.42±4.21 | 6.47±0.62 | 6s | >100 | >100 |
| 6g | 49.53±9.08 | 17.8±1.16 | 6t | >100 | >100 |
| 6h | 29.85±5.47 | 4.99±0.80 | 6u | >100 | 62.91±2.34 |
| 6i | 57.20±1.79 | 17.32±1.05 | 6v | 64.57±1.45 | 37.18±6.65 |
| 6j | 49.64±3.58 | 8.06±0.92 | 6w | >100 | 63.25±6.17 |
| 6k | 2.29±1.08 | 7.37±1.15 | vp-16a | 24.68±3.12 | 18.95±1.35 |
| 6l | 13.45±2.46 | 3.07±0.53 | CPTa | 0.34 | 0.012 |
| 6m | 72.43±2.15 | 4.79±0.73 | |||
| [1] |
Champoux, J. J. Annu. Rev. Biochem. 2001, 70, 369.
pmid: 11395412 |
| [2] |
Stewart, L.; Redinbo, M. R.; Qui, X.; Hol, W. G. J.; Champoux, J. J. Science 1998, 279, 1534.
pmid: 9488652 |
| [3] |
Berger, J. M.; Gamblin, S. J.; Harrison, S. C.; Wang, J. C. Nature 1996, 379, 225.
doi: 10.1038/379225a0 |
| [4] |
Lian, Q.; Xu, J.; Yan, S.; Huang, M.; Ding, H.; Sun, X.; Bi, A.; Ding, J.; Sun, B.; Geng, M. Cell Res. 2017, 27, 784.
doi: 10.1038/cr.2017.54 |
| [5] |
Pommier, Y. Nat. Rev. Cancer 2006, 6, 789.
pmid: 16990856 |
| [6] |
Thomas, A.; Pommier, Y. Clin. Cancer Res. 2019, 25, 6581.
doi: 10.1158/1078-0432.CCR-19-1089 pmid: 31227499 |
| [7] |
Newman, D. J.; Cragg, G. M. J. Nat. Prod. 2020, 83, 770.
doi: 10.1021/acs.jnatprod.9b01285 pmid: 32162523 |
| [8] |
Loiseleur, O. Chimia 2017, 71, 810.
doi: 10.2533/chimia.2017.810 pmid: 29289242 |
| [9] |
Lin, Z. Q.; Xia, W. L.; Liu, R. Y.; Jiang, S. H.; Ma, Z. Q. Chin. J. Org. Chem. 2020, 40, 2980. (in Chinese)
doi: 10.6023/cjoc202005006 |
|
(林芷晴, 夏婉铃, 刘仁义, 姜少华, 马志强, 有机化学, 2020, 40, 2980.)
doi: 10.6023/cjoc202005006 |
|
| [10] |
Rimando, A. M.; Cuendet, M.; Desmarchelier, C.; Mehta, R. G.; Pezzuto, J. M.; Duke, S. O. J. Agric. Food Chem. 2002, 50, 3453.
doi: 10.1021/jf0116855 |
| [11] |
Kimura, Y.; Okuda, H.; Arichi, S. Biochim. Biophys. Acta 1985, 834, 275.
pmid: 3922423 |
| [12] |
Stivala, L. A.; Savio, M.; Carafoli, F.; Perucca, P.; Bianchi, L.; Maga, G.; Forti, L.; Pagnoni, U. M.; Albini, A.; Prosperi, E.; Vannini, V. J. Biol. Chem. 2001, 276, 22586.
pmid: 11316812 |
| [13] |
Mahady, G. B.; Pendland, S. L.; Chadwick, L. R. Am. J. Gastroenterol. 2003, 98, 1440.
|
| [14] |
Shi, D.; An, R.; Zhang, W. B.; Zhang, G. L.; Yu, Z. G. J. Agric. Food Chem. 2017, 65, 60.
doi: 10.1021/acs.jafc.6b04303 |
| [15] |
Kronenwerth, M.; Dauth, C.; Kaiser, M.; Pemberton, I.; Bode, H. B. Eur. J. Org. Chem. 2014, 36, 8026.
|
| [16] |
Mizuno, C. S.; Schrader, K. K.; Rimando, A. M. J. Agric. Food Chem. 2008, 56, 9140.
doi: 10.1021/jf801988p |
| [17] |
Weng, J. Q.; Ali, A.; Estep, A.; Becnel, J.; Meyer, S. L. F.; Wedge, D. E.; Jacob, M.; Rimando, A. M. Chem. Biodiversity 2016, 13, 1165.
doi: 10.1002/cbdv.v13.9 |
| [18] |
Lu, Q.; Yu, Q.; Zhu, Y. B.; Weng, J. Q.; Yuan, J.; Hu, D. X.; Chen, J.; Liu, X. H.; Tan, C. X. J. Mol. Struct. 2019, 1180, 780.
doi: 10.1016/j.molstruc.2018.12.068 |
| [19] |
Mabkhot, Y. N.; Alharbi, M. M.; Al-Showiman, S. S.; Ghabbour, H. A.; Kheder, N. A.; Soliman, S. M.; Frey, W. Chem. Cent. J. 2018, 12, 56.
doi: 10.1186/s13065-018-0420-7 pmid: 29748782 |
| [20] |
Kouatly, O.; Geronikaki, A.; Kamoutsis, C.; Hadjipavlou-Litina, D.; Eleftheriou, P. Eur. J. Med. Chem. 2009, 44, 1198.
doi: 10.1016/j.ejmech.2008.05.029 pmid: 18603333 |
| [21] |
Pember, S. O.; Mejia, G. L.; Price, T. J.; Pasteris, R. J. Bioorg. Med. Chem. Lett. 2016, 26, 2965.
|
| [22] |
Koti, R. S.; Kolavi, G. D.; Hegde, V. S.; Khazi, I. M. Indian J. Chem. B 2006, 45, 1900.
|
| [23] |
Weng, J. Q.; Tan, C. X.; Liu, X. H. J. Pestic. Sci. 2012, 37, 164.
|
| [24] |
Yu, H. B.; Qin, Z. F.; Dai, H.; Zhang, X.; Qin, X.; Wang, T. T.; Fang, J. X. J. Agric. Food Chem. 2018, 56, 11356.
doi: 10.1021/jf802802g |
| [25] |
Weng, J. Q.; Liu, X. H.; Huang, H.; Tan, C. X.; Chen, J. Molecules 2012, 17, 989.
doi: 10.3390/molecules17010989 |
| [26] |
Zhang, J. H.; Zhu, Y. B.; Weng, J. Q.; Yu, Q.; Yuan, J.; Chen, J. Chin. J. Org. Chem. 2020, 40, 1055. (in Chinese)
doi: 10.6023/cjoc201910023 |
|
(章俊辉, 朱亚波, 翁建全, 余茜, 袁静, 陈杰, 有机化学, 2020, 40, 1055.)
doi: 10.6023/cjoc201910023 |
|
| [27] |
Kong, Y. L.; Xu, W. X.; Liu, X. H.; Weng, J. Q. Chin. Chem. Lett. 2020, 31, 3245.
doi: 10.1016/j.cclet.2020.05.022 |
| [28] |
Xu, W. X.; Ye, F. X.; Liu, X. H.; Weng, J. Q. Tetrahedron Lett. 2020, 61, 151807.
doi: 10.1016/j.tetlet.2020.151807 |
| [29] |
Dou, D. F.; He, G. J.; Li, Y.; Lai, Z.; Wei, L. Q.; Alliston, K. R.; Lushington, G. H.; Eichhorn, D. M.; Groutas, W. C. Bioorg. Med. Chem. 2010, 18, 1093.
doi: 10.1016/j.bmc.2009.12.057 |
| [30] |
Kocabas, E.; Sariguney, A. B.; Coskun, A. Heterocycles 2010, 81, 2849.
doi: 10.3987/COM-10-12067 |
| [31] |
Zhu, D. J.; Chen, J. X.; Wu, D. Z.; Liu, M. C.; Ding, J. C.; Wu, H. Y. J. Chem. Res. 2009, (2), 84.
|
| [32] |
Wetherill, J. P., Hann, R. M. J. Am. Chem. Soc. 1934, 56, 970.
doi: 10.1021/ja01319a068 |
| [33] |
Pommier, Y.; Covey, J. M.; Kerngan, D.; Markovits, J.; Pham, R. Nucleic Acids Res. 1987, 15, 6713.
pmid: 2819825 |
| [34] |
Staker, B. L.; Hjerrild, K.; Feese, M. D.; Behnke, C. A.; Burgin, A. B.; Jr.; Stewart, L. P. Natl. Acad. Sci. U. S. A. 2002, 99, 15387.
doi: 10.1073/pnas.242259599 |
| [1] | Fakai Zou, Nengzhong Wang, Hui Yao, Hui Wang, Mingguo Liu, Nianyu Huang. Regio- and Stereo-selective Synthesis of 1β-/3R-Aryl Thiosugar [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 593-604. |
| [2] | Luyao Li, Zhongwen He, Zhenguo Zhang, Zhenhua Jia, Teck-Peng Loh. Application of Triaryl Carbenium in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 421-437. |
| [3] | Qinggang Mei, Qinghan Li. Recent Progress of Visible Light-Induced the Synthesis of C(3) (Hetero)arylthio Indole Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 398-408. |
| [4] | Penghui Li, Qingyang Xie, Fuxian Wan, Yuanhong Zhang, Lin Jiang. Synthesis and Fungicidal Activity of Novel Substituted Pyrimidine-5-carboxamides Bearing Cyclopropyl Moiety [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 650-656. |
| [5] | Weiqing Yang, Yanbing Ge, Yuanyuan Chen, Ping Liu, Haiyan Fu, Menglin Ma. Design and Synthesis of Fluorescent 1,8-Napthalimide Derivatives and Their Identification of Cysteine [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 180-194. |
| [6] | Shihang Yu, Jiawei Liu, Biyu An, Qinghua Bian, Min Wang, Jiangchun Zhong. Asymmetric Synthesis of the Contact Sex Pheromone of Neoclytus acuminatus acuminatus (Fabricius) [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 301-308. |
| [7] | Xiaoying Jia, Jiaxia Pu, Lirong Han, Qinghan Li. Research Progress in the Synthesis of Benzo[d]pentamembered Heterocyclic Thioethers Containing Two Heteroatoms [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 18-40. |
| [8] | Qianfan Zhao, Yongzheng Chen, Shiming Zhang. Application and Mechanism Study of Carbon-Based Metal-Free Catalysts in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 137-147. |
| [9] | Shan Chen, Zhilin Chen, Qiong Hu, Yanshuang Meng, Yue Huang, Pingfang Tao, Liru Lu, Guobao Huang. Recognition of Bis-thiourea Tweezers to Neutral Molecules in Non-Polar Solvent [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 277-281. |
| [10] | Huakun Wang, Xiaolong Ren, Yining Xuan. Study of the Halide Salt Catalyzed [3+2] Cycloaddition of α,β-Epoxy Carboxylate with Isocyanate [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 251-258. |
| [11] | Yukun Jin, Baoyi Ren, Fushun Liang. Visible Light-Mediated Selective C—F Bond Cleavage of Trifluoromethyl Groups and Its Application in Synthesizing gem-Difluoro-Containing Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 85-110. |
| [12] | Cuiyun Ma, Hailan Luo, Fuhua Zhang, Dan Guo, Shuxing Chen, Fei Wang. Green Biosynthesis, Photophysical Properties and Application of 3-Pyrrolyl BODIPY [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 216-223. |
| [13] | Bozhen Wang, Jie Zhang, Chunhui Nian, Mingming Jin, Miaomiao Kong, Wulan Li, Wenfei He, Jianzhang Wu. Synthesis and Antitumor Activity of 3,4-Dichlorophenyl Amides [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 232-241. |
| [14] | Yang Li, Jinding Yuan, Di Zhao. Deep Eutectic Solvent of 1,3-Dimethylurea/L-(+)-Tartaric Acid for the Green Synthesis of (E)-2-Styrylquinoline-3-carboxylic Acid Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3268-3276. |
| [15] | Dandan Sui, Nannan Cen, Ruoqu Gong, Yang Chen, Wenbo Chen. Supporting-Electrolyte-Free Electrochemical Synthesis of Trifluoromethylated Oxindoles in Continuous Flow [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3239-3245. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||