Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (4): 1327-1336.DOI: 10.6023/cjoc202307015 Previous Articles Next Articles
ARTICLES
收稿日期:2023-07-15
修回日期:2023-09-12
发布日期:2023-11-23
基金资助:
Guodong Jua, Guangyu Zhoua, Yingsheng Zhaoa,b( )
)
Received:2023-07-15
Revised:2023-09-12
Published:2023-11-23
Contact:
E-mail: Supported by:Share
Guodong Ju, Guangyu Zhou, Yingsheng Zhao. Transition-Metal-Free Regioselective Thiocyanation of Triisopropylsilane (TIPS)-Protected Phenols[J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1327-1336.
| Entry | Oxidant | Solvent | [SCN] source | Yield/% |
|---|---|---|---|---|
| 1 | Oxone | HFIP | KSCN | 92 |
| 2 | Oxone | HFIP | AgSCN | NR |
| 3 | Oxone | HFIP | NH4SCN | 99 |
| 4 | K2S2O8 | HFIP | NH4SCN | 86 |
| 5 | H2O2 | HFIP | NH4SCN | 83 |
| 6 | O2 | HFIP | NH4SCN | Trace |
| 7 | TBHP | HFIP | NH4SCN | NR |
| 8 | Oxone | H2O | NH4SCN | NR |
| 9 | Oxone | DCE | NH4SCN | NR |
| 10 | Oxone | EtOH | NH4SCN | NR |
| 11 | Oxone | DMSO | NH4SCN | NR |
| 12 | Oxone | DMF | NH4SCN | NR |
| 13 | Oxone | Dioxane | NH4SCN | NR |
| 14 | Oxone | THF | NH4SCN | NR |
| 15 | Oxone | CH3CN | NH4SCN | NR |
| 16 | — | HFIP | NH4SCN | NR |
| Entry | Oxidant | Solvent | [SCN] source | Yield/% |
|---|---|---|---|---|
| 1 | Oxone | HFIP | KSCN | 92 |
| 2 | Oxone | HFIP | AgSCN | NR |
| 3 | Oxone | HFIP | NH4SCN | 99 |
| 4 | K2S2O8 | HFIP | NH4SCN | 86 |
| 5 | H2O2 | HFIP | NH4SCN | 83 |
| 6 | O2 | HFIP | NH4SCN | Trace |
| 7 | TBHP | HFIP | NH4SCN | NR |
| 8 | Oxone | H2O | NH4SCN | NR |
| 9 | Oxone | DCE | NH4SCN | NR |
| 10 | Oxone | EtOH | NH4SCN | NR |
| 11 | Oxone | DMSO | NH4SCN | NR |
| 12 | Oxone | DMF | NH4SCN | NR |
| 13 | Oxone | Dioxane | NH4SCN | NR |
| 14 | Oxone | THF | NH4SCN | NR |
| 15 | Oxone | CH3CN | NH4SCN | NR |
| 16 | — | HFIP | NH4SCN | NR |
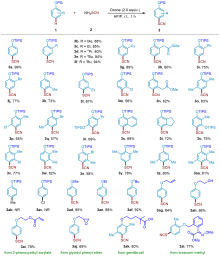
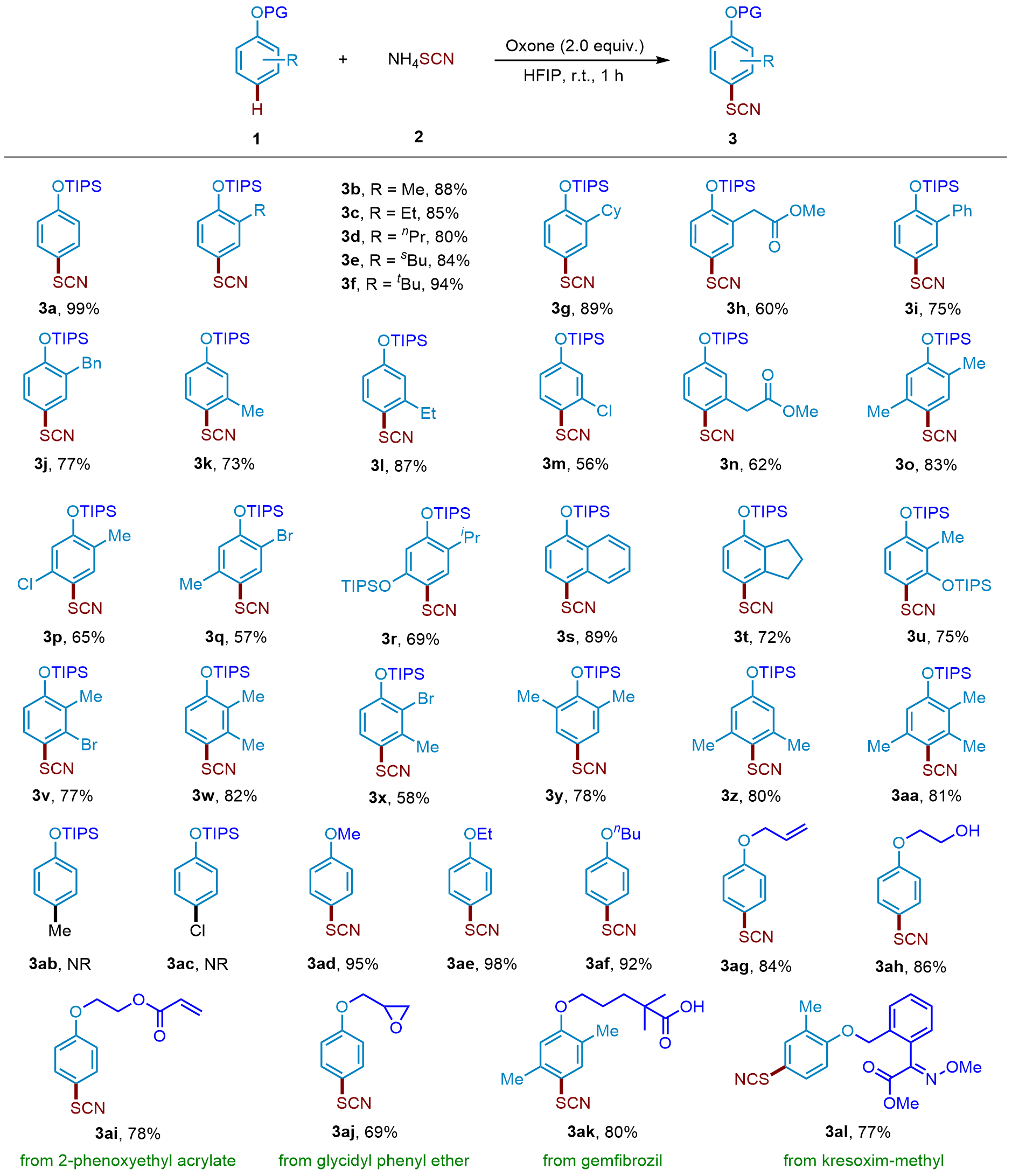
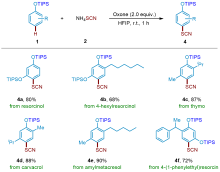
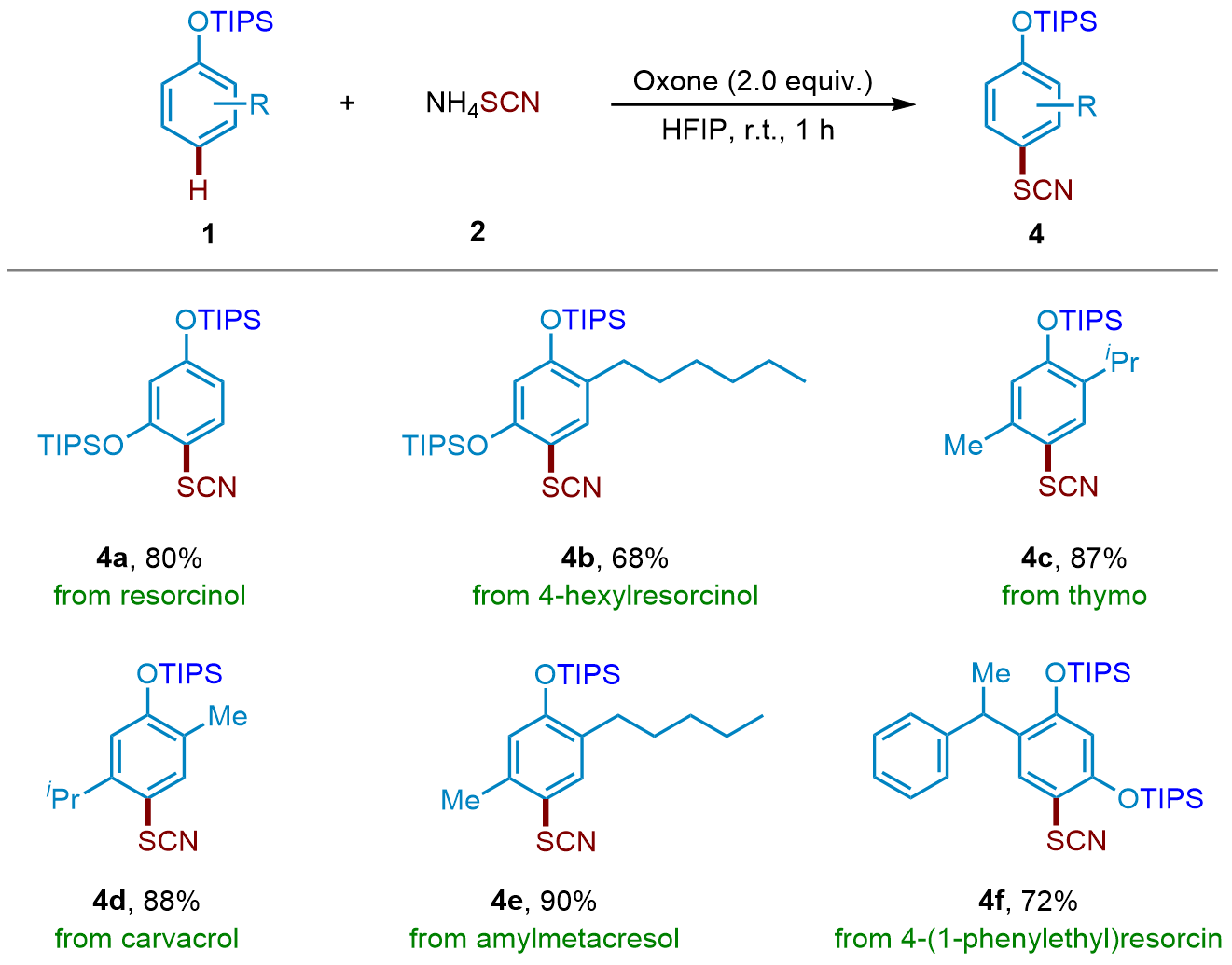
| [1] |
(a) Popoff I. C.; Engle A. R.; Whitaker R. L.; Shinghal G. H. J. Med. Chem. 1971, 14, 1166.
pmid: 25775431 |
|
(b) De Martino G.; Edler M. C.; La Regina G.; Coluccia A.; Barbera M. C.; Barrow D.; Nicholson R. I.; Chiosis G.; Brancale A.; Hamel E.; Artico M.; Silvestri R. J. Med. Chem. 2006, 49, 947.
doi: 10.1021/jm050809s pmid: 25775431 |
|
|
(c) Bagley M. C.; Davis T.; Dix M. C.; Fusillo V.; Pigeaux M.; Rokicki M. J.; Kipling D. J. Org. Chem. 2009, 74, 8336.
doi: 10.1021/jo9017155 pmid: 25775431 |
|
|
(d) Pawliczek M.; Garve L. K. B.; Werz D. B. Org. Lett. 2015, 17, 1716.
doi: 10.1021/acs.orglett.5b00494 pmid: 25775431 |
|
| [2] |
(a) Castanheiro T.; Suffert J.; Donnard M.; Gulea M. Chem. Soc. Rev. 2016, 45, 494.
doi: 10.1039/c5cs00532a pmid: 26658383 |
|
(b) Piña I. C.; Gautschi J. T.; Wang G.-Y.; Sanders M. L.; Schmitz F. J.; France D.; Cornell-Kennon S.; Sambucetti L. C.; Remiszewski S. W.; Perez L. B.; Bair K. W.; Crews P. J. Org. Chem. 2003, 68, 3866.
doi: 10.1021/jo034248t pmid: 26658383 |
|
| [3] |
Vaghasiya B. K.; Satasia S. P.; Thummar R. P.; Kamani R. D.; Avalani J. R.; Sapariya N. H.; Raval D. K. J. Sulfur Chem. 2018, 39, 507.
doi: 10.1080/17415993.2018.1469632 |
| [4] |
Danoun G.; Bayarmagnai B.; Gruenberg M. F.; Goossen L. J. J. Chem. Sci. 2014, 5, 1312.
|
| [5] |
Chen J.; Wang T.; Wang T.; Lin A.; Yao H.; Xu J. Org. Chem. Front. 2017, 4, 130.
doi: 10.1039/C6QO00590J |
| [6] |
Zhang T.; Deng G.; Li H.; Liu B.; Tan Q.; Xu B. Org. Lett. 2018, 20, 5439.
doi: 10.1021/acs.orglett.8b02347 pmid: 30106302 |
| [7] |
Yang L.; Tian Z.-Y.; Zhang C.-P. ChemistrySelect 2019, 4, 311.
doi: 10.1002/slct.v4.1 |
| [8] |
Segalovich-Gerendash G.; Rozenberg I.; Alassad N.; Nechmad N. B.; Goldberg I.; Kozuch S.; Lemcoff N. G. ACS Catal. 2020, 10, 4827.
doi: 10.1021/acscatal.0c00676 |
| [9] |
Grieco P. A.; Yokoyama Y.; Williams E. J. Org. Chem. 1978, 43, 12835.
|
| [10] |
Graßl S.; Hamze C.; Koller T. J.; Knochel P. Chem.-Eur. J. 2019, 25, 3752.
doi: 10.1002/chem.v25.15 |
| [11] |
Exner B.; Bayarmagnai B.; Matheis C.; Goossen L. J. J. Fluorine Chem. 2017, 198, 89.
doi: 10.1016/j.jfluchem.2016.12.006 |
| [12] |
Sun N.; H. Zhang, W.; Mo, B.; Hu, X. Synlett 2013, 24, 1443.
doi: 10.1055/s-00000083 |
| [13] |
Uemura S.; Onoe A, Okazaki H.; Okano M. Bull. Chem. Soc. Jpn. 1975, 48, 619.
doi: 10.1246/bcsj.48.619 |
| [14] |
Bacon R. G. R.; Guy R. G. J. Chem. Soc. 1960, 318.
|
| [15] |
Pan X.-Q.; Lei M.-Y.; Zou J.-P.; Zhang W. Tetrahedron Lett. 2009, 50, 347.
|
| [16] |
Kaufmann H. P.; Küchler K. Eur. J. Inorg. Chem. 1934, 67, 944.
|
| [17] |
(a) Fan W.; Yang Q.; Xu F.; Li P. J. Org. Chem. 2014, 79, 10588.
doi: 10.1021/jo5015799 |
|
(b) Mitra S.; Ghosh M.; Mishra S.; Hajra A. J. Org. Chem. 2015, 80, 8275.
doi: 10.1021/acs.joc.5b01369 |
|
|
(c) Zhang Z. G.; Liu X. X.; Zong X. L.; Yuan Y. L.; Liu S. L.; Zhang T.; Wu Z. S.; Yang J. Y. Chin. J. Org. Chem. 2021, 41, 52. (in Chinese)
doi: 10.6023/cjoc202008003 |
|
|
(张振国, 刘笑笑, 宗鑫龙, 苑亚林, 刘双磊, 张婷, 吴子尚, 杨静莹, 有机化学, 2021, 41, 52.)
doi: 10.6023/cjoc202008003 |
|
| [18] |
(a) Lin J.-P.; Zhang F.-H.; Long Y.-Q. Org. Lett. 2014, 16, 2822.
doi: 10.1021/ol500864r |
|
(b) Yang D.; Yan K.; Wei W.; Li G.; Lu S.; Zhao C.; Tian L.; Wang H. J. Org. Chem. 2015, 80, 11073.
|
|
|
(c) Fu Z. J.; Yang Z. J.; Z Sun L.; Yin J.; Yi X. Z.; Cai H.; Lei A. W. Chin. J. Org. Chem. 2022, 42, 600. (in Chinese)
doi: 10.6023/cjoc202107060 |
|
|
(付拯江, 杨振江, 孙丽, 尹健, 伊学政, 蔡琥, 雷爱文, 有机化学, 2022, 42, 600.)
doi: 10.6023/cjoc202107060 |
|
| [19] |
(a) Grant M. S.; Snyder H. R. J. Am. Chem. Soc. 1960, 82, 2742.
doi: 10.1021/ja01496a023 |
|
(b) Nair V.; George T. G.; Nair L. G.; Panicker S. B. Tetrahedron Lett. 1999, 40, 1195.
|
|
|
(c) Yadav J. S.; Reddy B. V. S.; Murali Krishna B. B. Synthesis 2008, 3779.
|
|
|
(d) Toste F. D.; Stefano V. D.; Still I. W. J. Synth. Commun. 1995, 25, 1277.
doi: 10.1080/00397919508012691 |
|
|
(e) Khazaei A.; Zolfigol M. A.; Mokhlesi M.; Pirveysian M. Can. J. Chem. 2012, 90, 427.
doi: 10.1139/v2012-013 |
|
|
(f) Zhang X.; Wang C.; Jiang H.; Sun L. RSC Adv. 2018, 8, 22042.
doi: 10.1039/C8RA04407D |
|
|
(g) Wu D.; Qiu J.; Karmaker P. G.; Yin H.; Chen F.-X. J. Org. Chem. 2018, 83, 1576.
doi: 10.1021/acs.joc.7b02850 |
|
|
(h) Wang Z.; Wang L.; Chen Q.; He M.-Y. Synth. Commun. 2018, 48, 76.
doi: 10.1080/00397911.2017.1390139 |
|
|
(i) Jiang H.; Yu W.; Tang X.; Li J.; Wu W. J. Org. Chem. 2017, 82, 9312.
doi: 10.1021/acs.joc.7b01122 |
|
| [20] |
(a) Mete T. B.; Khopade T. M.; Bhat R. G. T. Tetrahedron Lett. 2017, 58, 415.
doi: 10.1016/j.tetlet.2016.12.043 |
|
(b) Khalili D. New J. Chem. 2016, 40, 2547.
doi: 10.1039/C5NJ02314A |
|
|
(c) Murthy Y. L. N.; Govindh B.; Diwakar B. S.; Nagalakshmi K.; Venu R. J. Iran. Chem. Soc. 2011, 8, 292.
doi: 10.1007/BF03246227 |
|
|
(g) Khalili D. Chin. Chem. Lett. 2015, 26, 547.
doi: 10.1016/j.cclet.2015.01.007 |
|
|
(h) Wu G.; Liu Q.; Shen Y.; Wu W.; Wu L. Tetrahedron Lett. 2005, 46, 5831.
doi: 10.1016/j.tetlet.2005.06.132 |
|
|
(i) Yadav J. S.; Reddy B. V. S.; Shubashree S.; Sadashiv K. Tetrahedron Lett. 2004, 45, 2951.
doi: 10.1016/j.tetlet.2004.02.073 |
|
| [21] |
(a) Prakash O.; Kaur H.; Pundeer R.; Dhillon R. S.; Singh S. P. Synth. Commun. 2003, 33, 4037.
doi: 10.1081/SCC-120026343 pmid: 33403295 |
|
(b) de Oliveira Lima Filho E.; Malvestiti I. ACS Omega 2020, 5, 33329.
doi: 10.1021/acsomega.0c05131 pmid: 33403295 |
|
|
(c) Wang Z.; Wang L.; Chen Q.; He M.-Y. Synth. Commun. 2018, 48, 76.
doi: 10.1080/00397911.2017.1390139 pmid: 33403295 |
|
|
(d) Weng Z.; Wang H.; Wang L. Mendeleev Commun. 2023, 33, 118.
doi: 10.1016/j.mencom.2023.01.037 pmid: 33403295 |
|
| [22] |
(a) Geng J.; Fang Z.; Tu G.; Zhao Y. Chin. Chem. Lett. 2023, 34, 107609.
doi: 10.1016/j.cclet.2022.06.032 |
|
(b) Liu L. L.; Yang S.; Han Y.; Dai C. Y.; Shi D. Q.; Zhao Y. S. Chin. J. Org. Chem. 2020, 40, 2394. (in Chinese)
doi: 10.6023/cjoc202004019 |
|
|
(刘玲玲, 杨闪, 韩昳, 戴晨阳, 史达清, 黄志斌赵应声, 有机化学, 2020, 40, 2394.)
|
|
| [23] |
(a) Gao M.; Vuagnat M.; Chen M.-Y.; Pannecoucke X.; Jubault P.; Besset T. Chem.-Eur. J. 2021, 27, 6145.
doi: 10.1002/chem.v27.20 |
|
(b) Waddell L. J. N.; Senkans M. R.; Sutherland A. J. Org. Chem. 2023, 88, 7208.
doi: 10.1021/acs.joc.3c00454 |
|
| [24] |
Cernak T.; Dykstra K. D.; Tyagarajan S.; Vachal P.; Krska S. W. Chem. Soc. Rev. 2016, 45, 546.
doi: 10.1039/c5cs00628g pmid: 26507237 |
| [25] |
Munusamy S.; Muralidharan V. P.; Iyer S. K. Tetrahedron Lett. 2017, 58, 520.
doi: 10.1016/j.tetlet.2016.12.072 |
| [26] |
(a) Minisci F.; Citterio A.; Giordano C. Acc. Chem. Res. 1983, 16, 27.
doi: 10.1021/ar00085a005 |
|
(b) Hey D. H.; Jones G. H.; Perkins M. J. Chem. Soc., Perkin Trans. 1 1972, 118.
|
|
| [27] |
(a) Wu G.; Liu Q.; Shen Y.; Wu W.; Wu L. Tetrahedron Lett. 2005, 46, 5831.
doi: 10.1016/j.tetlet.2005.06.132 |
|
(b) Ali M.; Zarchi K.; Banihashemi R. Phosphorus, Sulfur Silicon Relat. Elem. 2014, 189, 1378.
doi: 10.1080/10426507.2013.865123 |
|
|
(c) Chen Y.; Chen C.; Liu Y.; Yu L. Chin. Chem. Lett. 2023, 34, 108489.
doi: 10.1016/j.cclet.2023.108489 |
| [1] | Yunhui Wan, Fumei Yang, Minghan Chen, Deli Sun, Danfeng Ye. Esterification of N-Benzyl-N-t-butoxycarbonyl-amides and Unsaturated Alcohol under Transition Metal-Free Conditions [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1293-1300. |
| [2] | Duyi Shen, Linghui Li, Ge Jing, Yujia Liang, Xinhui Zhang, Peiwei Gong, Fanjun Zhang, Mianran Chao. Advances in Flavin-Inspired Photocatalytic Oxidations Involving Single Electron Transfer Process [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1069-1093. |
| [3] | Jianghu Dong, Liangming Xuan, Chi Wang, Chenxi Zhao, Haifeng Wang, Qiongjiao Yan, Wei Wang, Fen'er Chen. Recent Advances in Visible-Light-Induced C(3)—H Functionalization of Quinoxalinones under Transition-Metal-Free or Photocatalyst-Free [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 111-136. |
| [4] | Zhongrong Xu, Jieping Wan, Yunyun Liu. Transition Metal-Free C—H Thiocyanation and Selenocyanation Based on Thermochemical, Photocatalytic and Electrochemical Process [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2425-2446. |
| [5] | Yijun Shi, Xinyue Sun, Han Cao, Fusheng Bie, Jie Ma, Zhe Liu, Xingshun Cong. Thioesterification of Esters with Primary Aliphatic Thiols at Room Temperature [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2499-2505. |
| [6] | Rui Wang, Lang Gao, Cen Zhou, Xiao Zhang. Haloperfluoroalkylation of Unactivated Terminal Alkenes over Phenylphenothiazine-Based Porous Organic Polymers [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1136-1145. |
| [7] | Qiyang Li, Haiyan Zhang, Wenbo Liu. Research Progress in Transition-Metal-Free C—Si Bond Formation [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3470-3490. |
| [8] | Wen-Chang Peng, Hui Wang, Dan-Wei Zhang, Zhan-Ting Li. Folding and Aggregation of Oligoviologens in Water and Cucurbit[n]uril (n=7, 8) Modulation [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 863-870. |
| [9] | Yu Zheng, Shencheng Qian, Pengcheng Xu, Binnan Zheng, Shenlin Huang. Electrochemical Oxidative Thiocyanosulfonylation of Aryl Acetylenes [J]. Chinese Journal of Organic Chemistry, 2022, 42(12): 4275-4281. |
| [10] | Huicheng Cheng, Penghu Guo, Bing Chen, Jiawei Yao, Jiaoli Ma, Weijie Hu, Hongbing Ji. Recent Advances in the Synthesis of Dibenzothiophenes [J]. Chinese Journal of Organic Chemistry, 2021, 41(1): 94-104. |
| [11] | Zhenguo Zhang, Xiaoxiao Liu, Xinlong Zong, Yalin Yuan, Shuanglei Liu, Ting Zhang, Zishang Wu, Jingying Yang, Zhenhua Jia. Recent Advance on the Synthesis of 3,3'-Bisindolylmethane Derivatives under Transition-Metal-Free Catalytic Conditions [J]. Chinese Journal of Organic Chemistry, 2021, 41(1): 52-64. |
| [12] | Zhang Longfei, Niu Cong, Yang Xiaoting, Qin Hongyun, Yang Jianjing, Wen Jiangwei, Wang Hua. Recent Advances on the Photocatalytic and Electrocatalytic Thiocyanation Reactions [J]. Chinese Journal of Organic Chemistry, 2020, 40(5): 1117-1128. |
| [13] | Wu Yan, Chen Jinyang, Li Qiang, Wei Wenting. Progress in C—N Bond Formation Involving C(sp2)—H Bond through Transition-Metal-Free Radical Reactions [J]. Chinese Journal of Organic Chemistry, 2020, 40(3): 589-597. |
| [14] | Xie Jianwei, Shen Li, Zhang Jie, Gong Shaofeng. Transition-Metal-Free Decarboxylative Amidation of Aryl α-Keto Acids with Diphenylphosphoryl Azide: New Avenue for the Preparation of Primary Aryl Amides [J]. Chinese Journal of Organic Chemistry, 2020, 40(12): 4284-4289. |
| [15] | Ren Linjing, Ran Maogang, He Jiaxin, Qian Yan, Yao Qiuli. Recent Advance in the Transition-Metal Free Coupling Reactions for the Construction of C-X Bonds Induced by Light [J]. Chin. J. Org. Chem., 2019, 39(6): 1583-1595. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||