Acta Chimica Sinica ›› 2019, Vol. 77 ›› Issue (9): 879-883.DOI: 10.6023/A19050193 Previous Articles Next Articles
Special Issue: 有机自由基化学
Communication
投稿日期:2019-05-25
发布日期:2019-07-01
通讯作者:
徐海超
E-mail:haichao.xu@xmu.edu.cn
基金资助:
Qian, Xiangyang, Xiong, Peng, Xu, Hai-Chao*( )
)
Received:2019-05-25
Published:2019-07-01
Contact:
Xu, Hai-Chao
E-mail:haichao.xu@xmu.edu.cn
Supported by:Share
Qian, Xiangyang, Xiong, Peng, Xu, Hai-Chao. Modular Synthesis of Functionalized 4-Quinolones via a Radical Cyclization Cascade Reaction[J]. Acta Chimica Sinica, 2019, 77(9): 879-883.
| Entry | Deviation from the standard conditions | Yield b/% |
|---|---|---|
| 1 | none | 96 |
| 2 | PhI(OAc)2 as oxidant | 0 (79) |
| 3 | I2O5 as oxidant | 0 |
| 4 | DMP as oxidant | 17 (28) |
| 5 | MeCN as solvent | 20 (63) |
| 6 | DMF as solvent | 0 (88) |
| 7 | DMSO/THF (1:10) as solvent | 51 (25) |
| 8 | CO (balloon) | 0 (39) |
| 9 | 1.0 gram of 1a | 80 |
| Entry | Deviation from the standard conditions | Yield b/% |
|---|---|---|
| 1 | none | 96 |
| 2 | PhI(OAc)2 as oxidant | 0 (79) |
| 3 | I2O5 as oxidant | 0 |
| 4 | DMP as oxidant | 17 (28) |
| 5 | MeCN as solvent | 20 (63) |
| 6 | DMF as solvent | 0 (88) |
| 7 | DMSO/THF (1:10) as solvent | 51 (25) |
| 8 | CO (balloon) | 0 (39) |
| 9 | 1.0 gram of 1a | 80 |
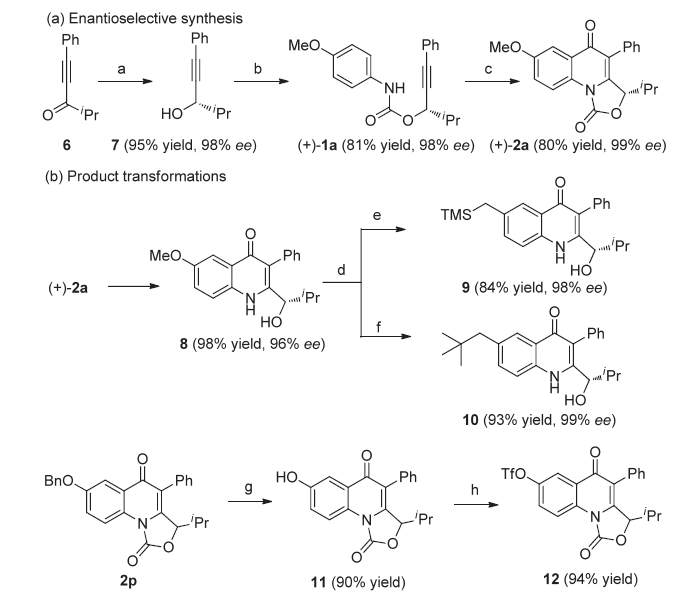
| [1] |
(a) Mitscher, L. A . Chem. Rev. 2005, 105, 559
doi: 10.1021/cr030101q |
|
(b) Zhanel, G. G.; Ennis, K.; Vercaigne, L.; Walkty, A.; Gin, A. S.; Embil, J.; Smith, H.; Hoban1, D. J . Drugs 2002, 62, 13.
doi: 10.1021/cr030101q |
|
| [2] |
(a) Nilsen, A.; Miley, G. P.; Forquer, I. P.; Mather, M. W.; Katneni, K.; Li, Y.; Pou, S.; Pershing, A. M.; Stickles, A. M.; Ryan, E.; Kelly, J. X.; Doggett, J. S.; White, K. L.; Hinrichs, D. J.; Winter, R. W.; Charman, S. A.; Zakharov, L. N.; Bathurst, I.; Burrows, J. N.; Vaidya, A. B.; Riscoe, M. K. J. Med. Chem. 2014, 57, 3818
doi: 10.1021/jm500147k |
|
(b) Nilsen, A.; LaCrue, A. N.; White, K. L.; Forquer, I. P.; Cross, R. M.; Marfurt, J.; Mather, M. W.; Delves, M. J.; Shackleford, D. M.; Saenz, F. E.; Morrisey, J. M.; Steuten, J.; Mutka, T.; Li, Y.; Wirjanata, G.; Ryan, E.; Duffy, S.; Kelly, J. X.; Sebayang, B. F.; Zeeman, A.-M.; Noviyanti, R.; Sinden, R. E.; Kocken, C. H. M.; Price, R. N.; Avery, V. M.; Angulo-Barturen, I.; Jiménez-Díaz, M. B.; Ferrer, S.; Herreros, E.; Sanz, L. M.; Gamo, F.-J.; Bathurst, I.; Burrows, J. N.; Siegl, P.; Guy, R. K.; Winter, R. W.; Vaidya, A. B.; Charman, S. A.; Kyle, D. E.; Manetsch, R.; Riscoe, M. K. Sci. Transl. Med. 2013, 5, 177ra137.
doi: 10.1021/jm500147k |
|
| [3] | Wang, Z. ( 2010). Conrad-Limpach Quinoline Synthesis. In Comprehensive Organic Name Reactions and Reagents, Z. Wang (Ed.). doi: 10.1002/9780470638859.conrr152. |
| [4] |
Gould, R. G.; Jacobs, W. A. J. Am. Chem. Soc. 1939, 61, 2890.
doi: 10.1021/ja01265a088 |
| [5] |
(a) Mukhina, O. A; Kutateladze, A. G. J. Am. Chem. Soc. 2016, 138, 2110
doi: 10.1021/jacs.5b12690 |
|
(b) Kwon, S.; Kang, D.; Hong, S. Eur. J. Org. Chem. 2015, 2015, 3671;
doi: 10.1021/jacs.5b12690 |
|
|
(c) Shao, T.; Jiang, Z . Acta Chim. Sinica 2017, 75, 70.
doi: 10.1021/jacs.5b12690 |
|
|
(d) Qiang Xie, Q.; Chen, X.-J.; Huang, P.-Q . Acta Chim. Sinica 2015, 73, 705.
doi: 10.1021/jacs.5b12690 |
|
| [6] |
(a) Malacria, M. Chem. Rev. 1996, 96, 289
doi: 10.1021/cr9500186 |
|
(b) Curran, D. P. Aldrichimica Acta 2000, 33, 104.
doi: 10.1021/cr9500186 |
|
| [7] |
Fuentes, N.; Kong, W. Q.; Fernandez-Sanchez, L.; Merino, E.; Nevado, C. J. Am. Chem. Soc. 2015, 137, 964.
doi: 10.1021/ja5115858 |
| [8] |
(a) Hou, Z. W.; Mao, Z. Y.; Zhao, H. B.; Melcamu, Y. Y.; Lu, X.; Song, J.; Xu, H.-C. Angew. Chem., Int. Ed. 2016, 55, 9168
doi: 10.1002/anie.201602616 |
|
(b) Zhu, L.; Xiong, P.; Mao, Z. Y.; Wang, Y. H.; Yan, X.; Lu, X.; Xu, H.-C. Angew. Chem., Int. Ed. 2016, 55, 2226;
doi: 10.1002/anie.201602616 |
|
|
(c) Hou, Z.-W.; Mao, Z.-Y.; Song, J.; Xu, H.-C . ACS Catal. 2017, 5810;
doi: 10.1002/anie.201602616 |
|
|
(d) Hou, Z.-W.; Yan, H.; Song, J.-S.; Xu, H.-C . Chin. J. Chem. 2018, 36, 909;
doi: 10.1002/anie.201602616 |
|
|
(e) Hou, Z.-W.; Mao, Z.-Y.; Melcamu, Y. Y.; Lu, X.; Xu, H.-C . Angew. Chem., Int. Ed. 2018, 57, 1636;
doi: 10.1002/anie.201602616 |
|
|
(f) Xu, F.; Long, H.; Song, J.; Xu, H.-C . Angew. Chem., Int. Ed. 2019, 58, 9017;
doi: 10.1002/anie.201602616 |
|
|
(g) Long, H.; Song, J. S.; Xu, H. C . Org. Chem. Front. 2018, 5, 3129;
doi: 10.1002/anie.201602616 |
|
|
(h) Xiong, P.; Xu, H.-H.; Xu, H.-C . J. Am. Chem. Soc. 2017, 139, 2956.
doi: 10.1002/anie.201602616 |
|
| [9] |
Nicolaou, K. C.; Baran, P. S.; Zhong, Y. L.; Barluenga, S.; Hunt, K. W.; Kranich, R.; Vega, J. A. J. Am. Chem. Soc. 2002, 124, 2233.
doi: 10.1021/ja012126h |
| [10] |
Matsumura, K.; Hashiguchi, S.; Ikariya, T.; Noyori, R. J. Am. Chem. Soc. 1997, 119, 8738.
doi: 10.1021/ja971570a |
| [11] |
(a) Tobisu, M.; Chatani, N. Acc. Chem. Res. 2015, 48, 1717
doi: 10.1021/acs.accounts.5b00051 |
|
(b) Guan, B.-T.; Xiang, S.-K.; Wu, T.; Sun, Z.-P.; Wang, B.-Q.; Zhao, K.-Q.; Shi, Z.-J . Chem. Commun. 2008, 1437;
doi: 10.1021/acs.accounts.5b00051 |
|
|
(c) Leiendecker, M.; Hsiao, C.-C.; Guo, L.; Alandini, N.; Rueping, M . Angew. Chem., Int. Ed. 2014, 53, 12912;
doi: 10.1021/acs.accounts.5b00051 |
|
|
(d) Tobisu, M.; Takahira, T.; Chatani, N . Org. Lett. 2015, 17, 4352.
doi: 10.1021/acs.accounts.5b00051 |
|
| [12] |
(a) Matsubara, H.; Ryu, I.; Schiesser, C. H. J. Org. Chem. 2005, 70, 3610
doi: 10.1021/jo047868i |
|
(b) Uenoyama, Y.; Fukuyama, T.; Nobuta, O.; Matsubara, H.; Ryu, I. Angew. Chem., Int. Ed. 2005, 44, 1075;
doi: 10.1021/jo047868i |
|
|
(c) Fukuyama, T.; Nakashima, N.; Okada, T.; Ryu, I . J. Am. Chem. Soc. 2013, 135, 1006.
doi: 10.1021/jo047868i |
| [1] | Shenna Deng, Changchun Peng, Yunhong Niu, Yun Xu, Yunxiao Zhang, Xiang Chen, Hongmin Wang, Shanshan Liu, Xiao Shen. Radical Brook Rearrangement Mediated Olefin Difunctionalization Involving α-Fluoroalkyl-α-silyl Methanols [J]. Acta Chimica Sinica, 2024, 82(2): 119-125. |
| [2] | Jianqiang Chen, Gangguo Zhu, Jie Wu. Recent Advances in Nickel-Catalyzed Ring Opening Cross-Coupling of Aziridines [J]. Acta Chimica Sinica, 2024, 82(2): 190-212. |
| [3] | Jinglin Yi, Mao Chen. Photo-Induced Copolymerization of Chlorotrifluoroethylene and Methyl Isopropenyl Ether★ [J]. Acta Chimica Sinica, 2024, 82(2): 126-131. |
| [4] | Yaning Li, Xiaoyan Wang, Yong Tang. The Regulation of Stereoselectivity in Radical Polymerization★ [J]. Acta Chimica Sinica, 2024, 82(2): 213-225. |
| [5] | Shan Li, Junxin Lu, Jie Liu, Lvqi Jiang, Wenbin Yi. Electrochemical Synthesis of α-Fluoroalkylated Ketones using Sodium Fluoroalkylsulfinate [J]. Acta Chimica Sinica, 2024, 82(2): 110-114. |
| [6] | Ping Li, Qiyu Yang, Jing Zeng, Ran Zhang, Qiuyan Chen, Fei Yan. Effect of Fluorine Doping on the Performance of Reversible Solid Oxide Cells and Related Kinetic Studies [J]. Acta Chimica Sinica, 2024, 82(1): 36-45. |
| [7] | Xinpu Fu, Xiuling Wang, Weiwei Wang, Rui Si, Chunjiang Jia. Fabrication and Mechanism Study of Clustered Au/CeO2 Catalyst for the CO Oxidation Reaction★ [J]. Acta Chimica Sinica, 2023, 81(8): 874-883. |
| [8] | Jianchuan Liu, Cuiyan Li, Yaozu Liu, Yujie Wang, Qianrong Fang. Highly-Stable Two-Dimensional Bicarbazole-based sp2-Carbon-conjugated Covalent Organic Framework for Efficient Electrocatalytic Oxygen Reduction★ [J]. Acta Chimica Sinica, 2023, 81(8): 884-890. |
| [9] | Yanyan Ren, Xin Li, Yingfeng Han. Synthesis and Optical Property Studies of Blue-Light Organic Radicals Based on N-Heterocyclic Carbenes★ [J]. Acta Chimica Sinica, 2023, 81(7): 735-740. |
| [10] | Li Liu, Gang Zheng, Guoqiang Fan, Hongguang Du, Jiajing Tan. Research Progress in Organic Reactions Involving 4-Acyl/Carbamoyl/Alkoxycarbonyl Substituted Hantzsch Esters [J]. Acta Chimica Sinica, 2023, 81(6): 657-668. |
| [11] | Wang Rui-Xiang, Zhao Qing-Ru, Gu Qing, You Shu-Li. Gold/Iridium Catalyzed Alkynylamide Cyclization/Asymmetric Allylic Benzylation Cascade Reaction★ [J]. Acta Chimica Sinica, 2023, 81(5): 431-434. |
| [12] | Xu Yuanli, Pan Hui, Yang Yi, Zuo Zhiwei. Selectively Aerobic Oxidation of Benzylic C—H Bonds Enabled by Dual Anthracene and Cerium Catalysis under Continuous-Flow Conditions★ [J]. Acta Chimica Sinica, 2023, 81(5): 435-440. |
| [13] | Huang Jiapian, Liu Fei, Wu Jie. Recent Advances in the Transformation of Difluorocyclopropenes★ [J]. Acta Chimica Sinica, 2023, 81(5): 520-532. |
| [14] | Huiying Zhang, Shuyan Yu, Congju Li. Electrocatalytic Degradation of Wastewater by Polymer-based Carbon Nanomembranes and Mechanism [J]. Acta Chimica Sinica, 2023, 81(4): 420-430. |
| [15] | Jie Yang, Lin Ling, Yuxue Li, Long Lu. Density Functional Theory Study on Thermal Decomposition Mechanisms of Ammonium Perchlorate [J]. Acta Chimica Sinica, 2023, 81(4): 328-337. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||