有机化学 ›› 2022, Vol. 42 ›› Issue (3): 871-883.DOI: 10.6023/cjoc202108031 上一篇 下一篇
研究论文
王秀a, 段文贵a,*( ), 林桂汕a,*(
), 林桂汕a,*( ), 李宝谕a, 张文静a, 雷福厚b
), 李宝谕a, 张文静a, 雷福厚b
收稿日期:2021-08-18
修回日期:2021-10-07
发布日期:2021-11-03
通讯作者:
段文贵, 林桂汕
基金资助:
Xiu Wanga, Wengui Duana( ), Guishan Lina(
), Guishan Lina( ), Baoyu Lia, Wenjing Zhanga, Fuhou Leib
), Baoyu Lia, Wenjing Zhanga, Fuhou Leib
Received:2021-08-18
Revised:2021-10-07
Published:2021-11-03
Contact:
Wengui Duan, Guishan Lin
Supported by:文章分享
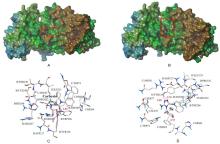
为了探寻以天然可再生资源为基础的生物活性分子, 设计并合成了24个新型含天然蒎烯结构的4-酰基-3-氨基- 1,2,4-三唑-硫醚衍生物, 通过IR, 1H NMR, 13C NMR, ESI-MS和元素分析对所有目标化合物的结构进行了表征. 初步的离体抑菌活性测试结果表明, 在质量浓度为50 μg/mL时, 目标化合物对测试的8种植物病原菌表现出一定的抑菌活性, 其中有4个化合物对苹果轮纹病菌有优良的抑菌活性, 远优于阳性对照百菌清; 2个化合物对花生褐斑病菌有良好的抑制活性, 远优于阳性对照百菌清. 使用比较分子力场分析法(CoMFA)对目标化合物的抗苹果轮纹病菌活性进行了初步的三维定量构效关系(3D-QSAR)分析, 建立了一个合理的3D-QSAR模型(r2=0.961, q2=0.613). 此外, 分子对接结果表明, 目标化合物诺卜醇基4-(4'-氟苯甲酰基)-3-氨基-1,2,4-三唑-硫醚(5k)在琥珀酸脱氢酶(SDH)活性口袋的结合模式与商品杀菌剂萎锈灵相似.
王秀, 段文贵, 林桂汕, 李宝谕, 张文静, 雷福厚. 含天然蒎烯结构的4-酰基-3-氨基-1,2,4-三唑-硫醚衍生物的合成、抑菌活性、三维定量构效关系及分子对接研究[J]. 有机化学, 2022, 42(3): 871-883.
Xiu Wang, Wengui Duan, Guishan Lin, Baoyu Li, Wenjing Zhang, Fuhou Lei. Synthesis, Antifungal Activity, Three-Dimensional Quantitative Structure-Activity Relationship and Molecular Docking Study of 4-Acyl-3-amino-1,2,4-triazole-thioether Derivatives Containing Natural Pinene Structure[J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 871-883.
| Compd. | Inhibition rate/% | |||||||
|---|---|---|---|---|---|---|---|---|
| F. oxysporum f. sp. Cucumerinum | C. arachidicola | P. piricola | A. solani | G. zeae | R. solani | B. myadis | C. orbicalare | |
| 5a | 24.5 | 48.6 | 45.0 | 41.4 | 58.7 | 63.5 | 43.8 | 40.0 |
| 5b | 42.2 | 82.9 | 83.0 | 66.2 | 66.6 | 21.2 | 49.8 | 39.6 |
| 5c | 29.1 | 34.3 | 45.0 | 48.6 | 52.3 | 57.0 | 34.3 | 35.0 |
| 5d | 33.6 | 27.1 | 38.8 | 34.3 | 29.7 | 30.9 | 34.3 | 30.0 |
| 5e | 66.3 | 74.3 | 85.2 | 84.1 | 73.4 | 90.5 | 77.4 | 72.9 |
| 5f | 24.5 | 27.1 | 38.8 | 41.4 | 26.5 | 41.7 | 24.8 | 30.0 |
| 5g | 33.6 | 41.4 | 54.4 | 41.4 | 42.6 | 35.2 | 34.3 | 35.0 |
| 5h | 24.5 | 41.4 | 51.3 | 34.3 | 45.8 | 59.1 | 43.8 | 35.0 |
| 5i | 24.5 | 24.3 | 28.8 | 27.1 | 61.9 | 30.9 | 29.5 | 30.0 |
| 5j | 24.5 | 48.6 | 41.9 | 34.3 | 39.4 | 59.1 | 43.8 | 40.0 |
| 5k | 51.5 | 80.0 | 93.9 | 79.0 | 47.6 | 24.9 | 34.9 | 33.7 |
| 5l | 29.1 | 41.4 | 41.9 | 41.4 | 36.1 | 30.9 | 29.5 | 30.0 |
| 5m | 38.2 | 41.4 | 70.0 | 41.4 | 71.6 | 57.0 | 34.3 | 40.0 |
| 5n | 24.5 | 34.3 | 38.8 | 41.4 | 36.1 | 41.7 | 34.3 | 30.0 |
| 5o | 24.5 | 41.4 | 54.4 | 48.6 | 58.7 | 50.4 | 43.8 | 35.0 |
| 5p | 88.2 | 77.1 | 70.0 | 77.1 | 84.5 | 89.6 | 67.6 | 80.0 |
| 5q | 33.6 | 41.4 | 51.3 | 48.6 | 55.5 | 57.0 | 39.0 | 30.0 |
| 5r | 24.5 | 41.4 | 45.0 | 41.4 | 45.8 | 70.0 | 29.5 | 40.0 |
| 5s | 38.2 | 34.3 | 32.5 | 41.4 | 52.3 | 46.1 | 43.8 | 30.0 |
| 5t | 45.9 | 68.6 | 61.3 | 61.0 | 37.2 | 27.3 | 41.3 | 39.6 |
| 5u | 42.2 | 74.3 | 93.5 | 71.3 | 66.6 | 26.1 | 34.9 | 39.6 |
| 5u | 33.6 | 34.3 | 35.6 | 41.4 | 26.5 | 28.7 | 39.0 | 30.0 |
| 5w | 16.2 | 30.4 | 47.6 | 40.0 | 34.0 | 12.3 | 18.9 | 33.3 |
| 5x | 24.5 | 27.1 | 38.8 | 41.4 | 52.3 | 37.4 | 24.8 | 30.0 |
| Chlorothalonil | 100 | 73.3 | 75.0 | 73.9 | 73.1 | 96.1 | 90.4 | 91.3 |
| Compd. | Inhibition rate/% | |||||||
|---|---|---|---|---|---|---|---|---|
| F. oxysporum f. sp. Cucumerinum | C. arachidicola | P. piricola | A. solani | G. zeae | R. solani | B. myadis | C. orbicalare | |
| 5a | 24.5 | 48.6 | 45.0 | 41.4 | 58.7 | 63.5 | 43.8 | 40.0 |
| 5b | 42.2 | 82.9 | 83.0 | 66.2 | 66.6 | 21.2 | 49.8 | 39.6 |
| 5c | 29.1 | 34.3 | 45.0 | 48.6 | 52.3 | 57.0 | 34.3 | 35.0 |
| 5d | 33.6 | 27.1 | 38.8 | 34.3 | 29.7 | 30.9 | 34.3 | 30.0 |
| 5e | 66.3 | 74.3 | 85.2 | 84.1 | 73.4 | 90.5 | 77.4 | 72.9 |
| 5f | 24.5 | 27.1 | 38.8 | 41.4 | 26.5 | 41.7 | 24.8 | 30.0 |
| 5g | 33.6 | 41.4 | 54.4 | 41.4 | 42.6 | 35.2 | 34.3 | 35.0 |
| 5h | 24.5 | 41.4 | 51.3 | 34.3 | 45.8 | 59.1 | 43.8 | 35.0 |
| 5i | 24.5 | 24.3 | 28.8 | 27.1 | 61.9 | 30.9 | 29.5 | 30.0 |
| 5j | 24.5 | 48.6 | 41.9 | 34.3 | 39.4 | 59.1 | 43.8 | 40.0 |
| 5k | 51.5 | 80.0 | 93.9 | 79.0 | 47.6 | 24.9 | 34.9 | 33.7 |
| 5l | 29.1 | 41.4 | 41.9 | 41.4 | 36.1 | 30.9 | 29.5 | 30.0 |
| 5m | 38.2 | 41.4 | 70.0 | 41.4 | 71.6 | 57.0 | 34.3 | 40.0 |
| 5n | 24.5 | 34.3 | 38.8 | 41.4 | 36.1 | 41.7 | 34.3 | 30.0 |
| 5o | 24.5 | 41.4 | 54.4 | 48.6 | 58.7 | 50.4 | 43.8 | 35.0 |
| 5p | 88.2 | 77.1 | 70.0 | 77.1 | 84.5 | 89.6 | 67.6 | 80.0 |
| 5q | 33.6 | 41.4 | 51.3 | 48.6 | 55.5 | 57.0 | 39.0 | 30.0 |
| 5r | 24.5 | 41.4 | 45.0 | 41.4 | 45.8 | 70.0 | 29.5 | 40.0 |
| 5s | 38.2 | 34.3 | 32.5 | 41.4 | 52.3 | 46.1 | 43.8 | 30.0 |
| 5t | 45.9 | 68.6 | 61.3 | 61.0 | 37.2 | 27.3 | 41.3 | 39.6 |
| 5u | 42.2 | 74.3 | 93.5 | 71.3 | 66.6 | 26.1 | 34.9 | 39.6 |
| 5u | 33.6 | 34.3 | 35.6 | 41.4 | 26.5 | 28.7 | 39.0 | 30.0 |
| 5w | 16.2 | 30.4 | 47.6 | 40.0 | 34.0 | 12.3 | 18.9 | 33.3 |
| 5x | 24.5 | 27.1 | 38.8 | 41.4 | 52.3 | 37.4 | 24.8 | 30.0 |
| Chlorothalonil | 100 | 73.3 | 75.0 | 73.9 | 73.1 | 96.1 | 90.4 | 91.3 |
| Compd. | R | MW | AF | AF" | Residue |
|---|---|---|---|---|---|
| 5a | C6H5 | 368.17 | –2.65 | –2.68 | 0.03 |
| 5b | o-CH3C6H4 | 382.18 | –1.89 | –1.94 | 0.05 |
| 5c | m-CH3C6H4 | 382.18 | –2.67 | –2.79 | 0.12 |
| 5d | p-CH3C6H4 | 382.18 | –2.78 | –2.66 | –0.12 |
| 5e | o-CH3OC6H4 | 398.18 | –1.84 | –1.84 | 0.00 |
| 5f | m-CH3OC6H4 | 398.18 | –2.8 | –2.82 | 0.02 |
| 5g | p-CH3CH2OC6H4 | 412.19 | –2.54 | –2.63 | 0.09 |
| 5h | p-CH3CH2C6H4 | 396.20 | –2.58 | –2.63 | 0.05 |
| 5i | o-FC6H4 | 386.16 | –2.98 | –3.14 | 0.16 |
| 5j | m-FC6H4 | 386.16 | –2.73 | –2.71 | –0.02 |
| 5k | p-FC6H4 | 386.16 | –1.40 | –1.44 | 0.04 |
| 5m | m-ClC6H4 | 402.13 | –2.24 | –2.40 | 0.16 |
| 5o | m-BrC6H4 | 446.08 | –2.57 | –2.45 | –0.12 |
| 5p | p-BrC6H4 | 446.08 | –2.28 | –2.14 | –0.14 |
| 5q | p-NCC6H4 | 393.16 | –2.57 | –2.61 | 0.04 |
| 5r | o-O2NC6H4 | 413.15 | –2.70 | –2.75 | 0.05 |
| 5t | p-CF3C6H4 | 436.15 | –2.44 | –2.45 | 0.01 |
| 5l* | o-ClC6H4 | 402.13 | –2.75 | –2.49 | –0.26 |
| 5n* | o-BrC6H4 | 446.08 | –2.85 | –2.51 | –0.34 |
| 5s* | p-O2NC6H4 | 413.15 | –2.93 | –2.68 | –0.25 |
| 5u* | o,m-Cl2C6H3 | 436.09 | –1.48 | –1.73 | 0.25 |
| 5v* | o,o,p-(CH3)3C6H2 | 410.21 | –2.87 | –3.08 | 0.21 |
| Compd. | R | MW | AF | AF" | Residue |
|---|---|---|---|---|---|
| 5a | C6H5 | 368.17 | –2.65 | –2.68 | 0.03 |
| 5b | o-CH3C6H4 | 382.18 | –1.89 | –1.94 | 0.05 |
| 5c | m-CH3C6H4 | 382.18 | –2.67 | –2.79 | 0.12 |
| 5d | p-CH3C6H4 | 382.18 | –2.78 | –2.66 | –0.12 |
| 5e | o-CH3OC6H4 | 398.18 | –1.84 | –1.84 | 0.00 |
| 5f | m-CH3OC6H4 | 398.18 | –2.8 | –2.82 | 0.02 |
| 5g | p-CH3CH2OC6H4 | 412.19 | –2.54 | –2.63 | 0.09 |
| 5h | p-CH3CH2C6H4 | 396.20 | –2.58 | –2.63 | 0.05 |
| 5i | o-FC6H4 | 386.16 | –2.98 | –3.14 | 0.16 |
| 5j | m-FC6H4 | 386.16 | –2.73 | –2.71 | –0.02 |
| 5k | p-FC6H4 | 386.16 | –1.40 | –1.44 | 0.04 |
| 5m | m-ClC6H4 | 402.13 | –2.24 | –2.40 | 0.16 |
| 5o | m-BrC6H4 | 446.08 | –2.57 | –2.45 | –0.12 |
| 5p | p-BrC6H4 | 446.08 | –2.28 | –2.14 | –0.14 |
| 5q | p-NCC6H4 | 393.16 | –2.57 | –2.61 | 0.04 |
| 5r | o-O2NC6H4 | 413.15 | –2.70 | –2.75 | 0.05 |
| 5t | p-CF3C6H4 | 436.15 | –2.44 | –2.45 | 0.01 |
| 5l* | o-ClC6H4 | 402.13 | –2.75 | –2.49 | –0.26 |
| 5n* | o-BrC6H4 | 446.08 | –2.85 | –2.51 | –0.34 |
| 5s* | p-O2NC6H4 | 413.15 | –2.93 | –2.68 | –0.25 |
| 5u* | o,m-Cl2C6H3 | 436.09 | –1.48 | –1.73 | 0.25 |
| 5v* | o,o,p-(CH3)3C6H2 | 410.21 | –2.87 | –3.08 | 0.21 |
| Statistical parameters | CoMFA |
|---|---|
| r2 a | 0.961 |
| q2 b | 0.613 |
| S c | 0.016 |
| F-valued | 124.113 |
| ONCe | 5 |
| Field contribution/% | CoMFA |
| Steric | 78.2 |
| Electrostatic | 21.8 |
| Statistical parameters | CoMFA |
|---|---|
| r2 a | 0.961 |
| q2 b | 0.613 |
| S c | 0.016 |
| F-valued | 124.113 |
| ONCe | 5 |
| Field contribution/% | CoMFA |
| Steric | 78.2 |
| Electrostatic | 21.8 |

| [1] |
Wang, H. Y.; Gao, X. H.; Zhang, X. X.; Jin, H.; Tao, K.; Hou, T. P. Bioorg. Med. Chem. Lett. 2017, 27, 90.
doi: 10.1016/j.bmcl.2016.11.026 |
| [2] |
Qi, L.; Li, M. C.; Bai, J. C.; Ren, Yi. H.; Ma, H. X. Bioorg. Med. Chem. Lett. 2021, 40, 127902.
doi: 10.1016/j.bmcl.2021.127902 |
| [3] |
Yu, B.; Zhou, S.; Cao, L. X.; Hao, Z. S.; Yang, D. Y.; Guo, X. F.; Zhang, N. L.; Bakulev, V. A.; Fan, Z. J. J. Agric. Food Chem. 2020, 68, 7093.
doi: 10.1021/acs.jafc.0c00062 |
| [4] |
Yoshikawa, Y.; Katsuta, H.; Kishi, J.; Yanase, Y. J. Pestic. Sci. 2011, 36, 347.
doi: 10.1584/jpestics.G10-70 |
| [5] |
FRAC classification of fungicides. http://www.frac.info/(accessed 12 Aug 2021).
|
| [6] |
García, D.; Bustamante, F.; Villa, A. L.; Lapuerta, M.; Alarcon, E. Energy Fuels 2020, 34, 579.
doi: 10.1021/acs.energyfuels.9b03742 |
| [7] |
Nie, G. K.; Zou, J. J.; Feng, R.; Zhang, X. W.; Wang, L. Catal. Today 2014, 234, 271.
doi: 10.1016/j.cattod.2013.12.003 |
| [8] |
Xu, B.; Zhai, Q. H.; Li, Z. X.; Zheng, G. Y. Chem. Ind. Forest. Prod. 1992, 1, 75. (in Chinese)
|
|
(许彬, 翟其骅, 李仲训, 郑光耀, 林产化学与工业, 1992, 1, 75.)
|
|
| [9] |
Jadhav, S. V.; Jinka, K. M.; Bajaj, H. C. Appl. Catal. A: Gen. 2010, 390, 158.
doi: 10.1016/j.apcata.2010.10.005 |
| [10] |
Vrbková, E.; Štefová, B.; Vyskočilová, E.; Červený, L. React. Kinet., ech. Catal. 2020, 131, 213.
|
| [11] |
Chen, J. Z.; Xiao, Z. Q.; Xu, L. F.; Wang, Z. D. Chem. Res. Appl. 2017, 29, 1728. (in Chinese)
|
|
(陈金珠, 肖转全, 徐丽锋, 王宗德, 化学研究与应用, 2017, 29, 1728.)
|
|
| [12] |
Nyamwihura, R. J.; Zhang, H. S.; Collins, J. T.; Crown, O.; Ogungbe, I. V. Molecules 2021, 26, 1008.
doi: 10.3390/molecules26041008 |
| [13] |
Jin, L. L.; Xiao, Z. Q.; Chen, J. Z.; Fan, G. R.; Wang, P.; Wang, Z. D.; Chen, S. X. J. Jiangxi Normal Univ. Nat. Sci. 2015, 39, 484. (in Chinese)
|
|
(金霖霖, 肖转全, 陈金珠, 范国荣, 王鹏, 王宗德, 陈尚钘, 江西师范大学学报(自然科学版), 2015, 39, 484.)
|
|
| [14] |
Han, Z. J.; Wang, Z. D.; Jiang, Z. K.; Zheng, W. Q.; Qian, W. H.; Chen, J. Z.; Chen, C. Acta Agric. Univ. Jiangxiensis 2008, 30, 586. (in Chinese)
|
|
(韩招久, 王宗德, 姜志宽, 郑卫青, 钱万红, 陈金珠, 陈超, 江西农业大学学报, 2008, 30, 586.)
|
|
| [15] |
Han, Z. J.; Wang, Z. D.; Jiang, Z. K.; Jin, X. Y.; Qian, W. H.; Chen, C.; Chen, J. Z.; Zheng, W. Q. Chin. Bull. Entomol. 2007, 44, 863. (in Chinese)
|
|
(韩招久, 王宗德, 姜志宽, 金宪杨, 钱万红, 陈超, 陈金珠, 郑卫青, 昆虫知识, 2007, 44, 863.)
|
|
| [16] |
He, L. L.; Tian, Q.; Yang, Q.; Zhu, X. M.; Yang, J. S. Chem. Res. Appl. 2008, 20, 600. (in Chinese)
|
|
(何丽丽, 田强, 杨倩, 朱星枚, 杨劲松, 化学研究与应用, 2008, 20, 600.)
|
|
| [17] |
Chen, M.; Duan, W. G.; Lin, G. S.; Fan, Z. T.; Wang, X. Molecules 2021, 26, 1708.
doi: 10.3390/molecules26061708 |
| [18] |
Wang, X.; Duan, W. G.; Lin, G. S.; Chen, M.; Lei, F. H. Res. Chem. Intermed. 2021, 47, 4029.
doi: 10.1007/s11164-021-04510-x |
| [19] |
Amin, N. H.; El-Saadi, M. T.; Ibrahim, A. A.; Abdel-Rahman H. M. Bioorg. Chem. 2021, 111, 104841.
doi: 10.1016/j.bioorg.2021.104841 |
| [20] |
Bitla, S.; Gayatri, A. A.; Puchakayala, M. R.; Bhukya, V. K.; Vannada, J.; Dhanavath, R.; Kuthati, B.; Kothula, D.; Sagurthi, S. R.; Atcha, K. R. Bioorg. Med. Chem. Lett. 2021, 41, 128004.
doi: 10.1016/j.bmcl.2021.128004 |
| [21] |
Shahzadi, I.; Zahoor, A. F.; Rasul, A.; Mansha, A.; Ahmad, S.; Raza, Z. ACS Omega 2021, 6, 11943.
doi: 10.1021/acsomega.1c00424 pmid: 34056349 |
| [22] |
Chen, Y.; Li, P.; Su, S. J.; Chen, M.; He, J.; Liu, L. W.; He, M. Wang, H.; Xue, W. RSC Adv. 2019, 9, 23045.
doi: 10.1039/C9RA05139B |
| [23] |
Abdel-Aziz, M.; Beshr, E. A.; Abdel-Rahman, I. M.; Ozadali, K.; Tan, O. U.; Aly, O. M. Eur. J. Med. Chem. 2014, 77, 155.
doi: 10.1016/j.ejmech.2014.03.001 pmid: 24631895 |
| [24] |
Peng, Z. Y.; Wang, G. C.; Zeng, Q. H.; Li, Y. F.; Wu, Y.; Liu, H. Q.; Wang, J. J.; Zhao, Y. Food Chem. 2021, 341, 128265.
doi: 10.1016/j.foodchem.2020.128265 |
| [25] |
Liao, L. P.; Jiang, C. B.; Chen, J. W.; Shi, J. G.; Li, X. H.; Wang, Y.; Wen, J.; Zhou, S. J.; Liang, J.; Lao, Y. Q.; Zhang, J. X. Eur. J. Med. Chem. 2020, 190, 112114.
doi: 10.1016/j.ejmech.2020.112114 |
| [26] |
Mustafa, M.; Anwar, S.; Elgamal, F.; Ahmed, E. R.; Aly, O. M. Eur. J. Med. Chem. 2019, 183, 111697.
doi: 10.1016/j.ejmech.2019.111697 |
| [27] |
Ghosh, S.; Liu, Y.; Garg, G.; Anyika, Mercy.; McPherson, N. T.; Ma, J.; Dobrowsky, R. T.; Blagg, B. S. J. ACS Med. Chem. Lett. 2016, 7, 813.
doi: 10.1021/acsmedchemlett.6b00224 |
| [28] |
Choi, M.; Jo, H.; Kim, D.; Yun, J.; Kang, J. S.; Kim, Y.; Jung, J. K.; Hong, J. T.; Cho, J.; Kwak, J. H.; Lee, H. Arch. Pharm. Res. 2016, 39, 618.
doi: 10.1007/s12272-016-0737-5 |
| [29] |
Wu, W. N.; Lan, W. J.; Wu, C. Y.; Fei, Q. Front. Chem. DOI: 10.3389/fchem.2021.695628.
doi: 10.3389/fchem.2021.695628 |
| [30] |
Wei, Q. Y.; Wang, X. M.; Cheng, J. H.; Zeng, G. X.; Sun, D. W. Food Chem. 2018, 268, 220.
doi: 10.1016/j.foodchem.2018.06.071 |
| [31] |
Liu, J. B.; Li, Y. X.; Zhang, X. L.; Cheng, D. D.; Wei, W.; Wu, C. C.; Xie, Y. T.; Xiong, L. X.; Li, Z. M. Chin. J. Chem. 2017, 35, 368.
doi: 10.1002/cjoc.v35.3 |
| [32] |
Espinoza-Moraga, M.; Singh, K.; Njoroge, M.; Kaur, G. Okombo, J.; Kock, C. D.; Smith, P. J.; Wittlin, S.; Chibale, K. Bioorg. Med. Chem. Lett. 2017, 27, 658.
doi: S0960-894X(16)31241-0 pmid: 28012840 |
| [33] |
Narsinghani, T.; Sharma, R. Chem. Pap. 2017, 71, 857.
doi: 10.1007/s11696-016-0102-7 |
| [34] |
Xie, Y.; Ruan, X. H.; Gong, H. Y.; Wang, Y. H.; Wang, X. B.; Zhang, J. P.; Li, Q.; Xue, W. J. Heterocycl. Chem. 2017, 54, 2644.
doi: 10.1002/jhet.v54.5 |
| [35] |
Wang, X.; Pang, F. H.; Huang, L.; Yang, X. P.; Ma, X. L.; Jiang, C. N.; Li, F. Y.; Lei, F. H. Int. J. Mol. Sci. 2018, 19, 3116.
doi: 10.3390/ijms19103116 |
| [36] |
Zhu, X. P.; Lin, G. S.; Duan, W. G.; Li, Q. M.; Li, F. Y.; Lu, S. Z. Molecules 2020, 25, 986.
doi: 10.3390/molecules25040986 |
| [37] |
Yu, Y. P.; Duan, W. G.; Lin, G. S.; Kang, G. Q.; Wang, X. Y.; Lei, F. H. Chin. J. Org. Chem. 2020, 40, 1647. (in Chinese)
doi: 10.6023/cjoc201912042 |
|
(虞友培, 段文贵, 林桂汕, 康国强, 王晓宇, 雷福厚, 有机化学, 2020, 40, 1647.)
doi: 10.6023/cjoc201912042 |
|
| [38] |
Zhao, S. Y.; Lin, G. S.; Duan, W. G.; Zhang, Q. A.; Huang, Y. L.; Lei, F. H. ACS Omega 2021, 6, 9104.
doi: 10.1021/acsomega.1c00217 |
| [39] |
Liu, X. L.; Hu, T. T.; Lin, G. S.; Wang, X.; Zhu, Y.; Liang, R. Z.; Duan, W. G.; Wei, M. RSC Adv. 2020, 10, 9786.
doi: 10.1039/D0RA00572J |
| [40] |
Li, C. F.; Cen, B.; Duan, W. G.; Lin, G. S.; Wang, X.; Li, B. Y. Chin. J. Org. Chem. 2021, 41, 2485. (in Chinese)
doi: 10.6023/cjoc202011023 |
|
(李成飞, 岑波, 段文贵, 林桂汕, 王秀, 李宝谕, 有机化学, 2021, 41, 2485.)
doi: 10.6023/cjoc202011023 |
|
| [41] |
Yi, F. P.; Ke, M.; Wang, L. S. Chem. Ind. Forest. Prod. 2000, 20, 55. (in Chinese)
|
|
(易封萍, 柯敏, 王立升, 林产化学与工业, 2000, 20, 55.)
|
|
| [42] |
Balczewski, P.; Bachowska, B.; Bialas, T.; Biczak, R.; Wieczorek, W. M.; Balinäska, A. J. Agric. Food Chem. 2007, 55, 1881.
doi: 10.1021/jf062849q |
| [43] |
Huang, M.; Duan, W. G.; Lin, G. S.; Li, K.; Hu, Q. Molecules 2017, 22, 1538.
doi: 10.3390/molecules22091538 |
| [44] |
Dolzhenko, A. V.; Pastorin, G.; Dolzhenko, A. V.; Chui, W. K. Tetrahedron Lett. 2009, 50, 2124.
doi: 10.1016/j.tetlet.2009.02.172 |
| [45] |
Takács, M.; Bubenyák, M.; Váradi, A.; Blazics, B.; Horváth, P.; Kökösi, J. Tetrahedron Lett. 2011, 52, 1863.
doi: 10.1016/j.tetlet.2011.02.024 |
| [46] |
Tahlan, S.; Kumar, P.; Ramasamy, K.; Mani, V.; Mishra, R. K.; Majeed, A. B. A. Narasimhan, B. Arabian J. Chem. 2017, 10(Suppl 2), A57.
|
| [47] |
Wang, Z. D.; Xiao, Z. Q.; Chen, J. Z. Chem. World 2004, 45, 89. (in Chinese)
|
|
(王宗德, 肖转泉, 陈金珠, 化学世界, 2004, 45, 89.)
|
|
| [48] |
Yi, F. P.; Zhou, Y. H.; Li, W. G.; Liu, X. M. Chem. World 2001, 42, 93. (in Chinese)
|
|
(易封萍, 周永红, 李伟光, 刘雄民, 化学世界, 2001, 42, 93.)
|
|
| [49] |
Su, N. N.; Li, Y.; Yu, S. J.; Zhang, X.; Liu, X. H.; Zhao, W. G. Res Chem. Intermed. 2013, 39, 759.
doi: 10.1007/s11164-012-0595-9 |
| [50] |
Lin, G. S.; Bai, X.; Duan, W. G.; Cen, B.; Huang, M.; Lu, S. Z. ACS Sustainable Chem. Eng. 2019, 7, 7862.
doi: 10.1021/acssuschemeng.9b00254 |
| [51] |
Xiong, L.; Li, H.; Jiang, L. N.; Ge, J. M.; Yang, W. C.; Zhu, X. L.; Yang G. F. J. Agric. Food Chem. 2017, 65, 1021.
doi: 10.1021/acs.jafc.6b05134 |
| [52] |
Bai, H.; Liu, X. L.; Chenzhang, P. F.; Xiao, Y. M.; Fu, B.; Qin, Z. H. Molecules 2020, 25, 5852.
doi: 10.3390/molecules25245852 |
| [1] | 吴思敏, 唐嘉欣, 周于佳, 徐学涛, 张昊星, 王少华. 2β-Acetoxyferruginol去醋酸基骨架衍生物抑制α-葡萄糖苷酶活性研究[J]. 有机化学, 2024, 44(2): 613-621. |
| [2] | 霍海波, 李桂霞, 王世军, 韩春, 师宝君, 李健. 新型γ-咔啉衍生物的合成及其抑菌活性研究[J]. 有机化学, 2024, 44(1): 204-215. |
| [3] | 王锋, 陈钰, 裴鸿艳, 张静, 张立新. 含哌啶的新型1,2,4-噁二唑类衍生物的设计合成及抗真菌活性研究[J]. 有机化学, 2023, 43(8): 2826-2836. |
| [4] | 刘敏, 杨冬燕, 肖玉梅, 苏旺苍, 赵峰海, 覃兆海. 5-硝基亚氨基[1,4-2H]-1,2,4-三唑啉烯式吡虫啉类似物的合成及生物活性研究[J]. 有机化学, 2023, 43(8): 2790-2799. |
| [5] | 徐欢, 吴鸿飞, 张晓鸣, 路星星, 孙腾达, 亓悦, 林誉凡, 杨新玲, 张莉, 凌云. 含1,2,3,4-四氢异喹啉片段磺酰肼和酰肼类化合物的设计、合成及生物活性研究[J]. 有机化学, 2023, 43(2): 725-733. |
| [6] | 孙昌兴, 张福豪, 张欢, 李鹏辉, 姜林. 新型2-(1-甲基-1H-吡唑-4-基)嘧啶-4-甲酰胺的设计、合成、杀菌活性及分子对接研究[J]. 有机化学, 2023, 43(1): 229-235. |
| [7] | 汪蕾, 于淑晶, 杨娜, 王宝雷. 新型含二氢喹唑啉酮的咖啡因衍生物的合成及生物活性研究[J]. 有机化学, 2023, 43(1): 299-307. |
| [8] | 王长凯, 孙腾达, 张学博, 杨新玲, 路星星, 徐欢, 石发胜, 张莉, 凌云. 新型含氟吡唑酰肼类化合物的设计合成与生物活性研究[J]. 有机化学, 2022, 42(5): 1527-1536. |
| [9] | 孔媛芳, 杨彬, 庄严, 张京玉, 孙德梅, 董春红. 基于二肽基肽酶4 (DPP-4)靶点设计的五种降糖活性杂环合成及构效关系研究进展[J]. 有机化学, 2022, 42(3): 770-784. |
| [10] | 崔玉成, 陈美桦, 林桂汕, 段文贵, 李晴敏, 邹壬萱, 岑波. 含偕二甲基环丙烷结构的1,3,4-噻二唑-脲化合物的合成、抑菌活性及分子对接研究[J]. 有机化学, 2022, 42(11): 3784-3797. |
| [11] | 陈睿嘉, 周聪, 逄锡文, 刘佳君, 顾玉诚, 刘建文, 李忠. 以环丙烷限制构象的新型双酰胺的设计、合成、抗癌活性及计算分析[J]. 有机化学, 2022, 42(1): 277-292. |
| [12] | 徐洪亮, 苏静, 王子时, 侯晨忻, 吴鹏冲, 邢月, 李香帅, 朱晓磊, 路运才, 徐利剑. 苯基吡咯类杀菌剂的设计合成及三维-定量构效关系(3D-QSAR)研究[J]. 有机化学, 2021, 41(9): 3560-3570. |
| [13] | 李英俊, 林乐弟, 刘季红, 高立信, 盛丽, 靳焜, 刘雪洁, 杨鸿境, 李佳. 新型含咔唑环和芳环/芳稠杂环的N-酰腙衍生物的合成及蛋白酪氨酸磷酸酶1B (PTP1B)抑制活性评价[J]. 有机化学, 2021, 41(9): 3593-3607. |
| [14] | 李英俊, 林乐弟, 靳焜, 高立信, 盛丽, 刘季红, 李佳. 新型含咔唑环芳氨基乙酰腙衍生物的合成及其蛋白酪氨酸磷酸酶1B (PTP1B)抑制活性评价[J]. 有机化学, 2021, 41(8): 3157-3170. |
| [15] | 李成飞, 岑波, 段文贵, 林桂汕, 王秀, 李宝谕. 含天然苯乙烯结构的4-甲基-1,2,4-三唑-硫醚化合物的合成、除草活性及三维定量构效关系(3D-QSAR)研究[J]. 有机化学, 2021, 41(6): 2485-2495. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||