有机化学 ›› 2022, Vol. 42 ›› Issue (4): 1129-1135.DOI: 10.6023/cjoc202110025 上一篇 下一篇
研究论文
朱思玉a, 霍新玉a, 马芹b, 陈伟b, 张洁a,*( ), 郭亮a,*(
), 郭亮a,*( )
)
收稿日期:2021-10-18
修回日期:2021-12-23
发布日期:2022-01-11
通讯作者:
张洁, 郭亮
基金资助:
Siyu Zhua, Xinyu Huoa, Qin Mab, Wei Chenb, Jie Zhanga( ), Liang Guoa(
), Liang Guoa( )
)
Received:2021-10-18
Revised:2021-12-23
Published:2022-01-11
Contact:
Jie Zhang, Liang Guo
Supported by:文章分享
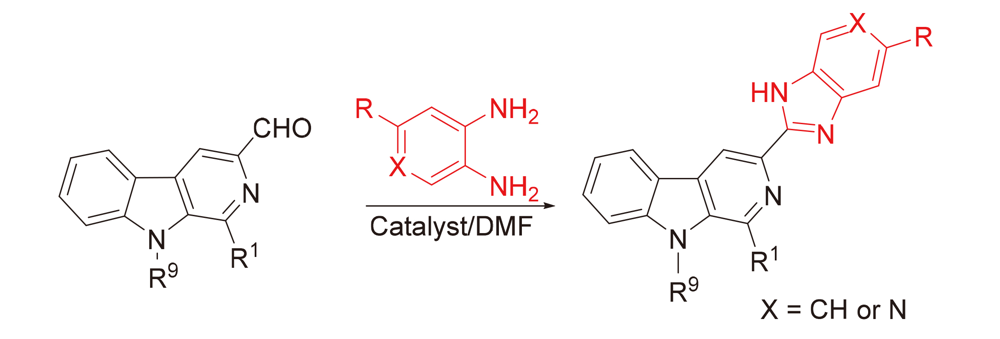
为进一步发现具有更好药理活性的新型β-咔啉类抗肿瘤药物, 以L-色氨酸和不同种类的醛为原料设计并合成了一系列具有不同取代基的1,9-二取代-β-咔啉-苯并咪唑偶联物. 采用噻唑蓝(MTT)法评估了目标化合物对肺癌(A549)、胃癌(BGC-823)、结肠癌(CT-26)、肝癌(Bel-7402)和乳腺癌(MCF-7)等五种肿瘤细胞株的体外抗肿瘤活性, 讨论了β-咔啉环1位和9位取代基对抗肿瘤活性的影响. 结果显示大多数目标化合物表现出广谱的抗肿瘤活性, 获得了初步的构效关系. 特别是化合物5s, 在β-咔啉环9位具有苄基且苯并咪唑环上具有三氟甲基取代, 对MCF-7细胞株具有最好的抗肿瘤活性, 其IC50值为(4.9±0.3) μmol/L, 抗肿瘤活性明显优于阳性对照药顺铂. 化合物5c和5q对测试的3株肿瘤细胞都表现出良好的活性且IC50值小于10 μmol/L; 细胞划痕实验结果表明这两个化合物对A549细胞横向迁移能力有一定的抑制效果
朱思玉, 霍新玉, 马芹, 陈伟, 张洁, 郭亮. β-咔啉-苯并咪唑偶联物的合成及抗肿瘤活性研究[J]. 有机化学, 2022, 42(4): 1129-1135.
Siyu Zhu, Xinyu Huo, Qin Ma, Wei Chen, Jie Zhang, Liang Guo. Design, Synthesis, and Antitumor Activity of β-Carboline-Benzimidazole Hybrids[J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1129-1135.

| Compd. | R1 | R9 | R | X | IC50/(μmol•L–1)±SDa | ||||
|---|---|---|---|---|---|---|---|---|---|
| A549 | BGC-823 | CT-26 | Bel-7402 | MCF-7 | |||||
| 5a | H | n-Butyl | H | CH | 32.1±2.8 | 16.1±1.4 | 17.2±1.3 | 13.3±1.1 | 12.7±0.9 |
| 5b | H | Benzyl | H | CH | 9.7±0.7 | 17.5±1.6 | 13.8±1.1 | 15.6±1.3 | 16.1±1.2 |
| 5c | H | 4-Fluorobenzyl | H | CH | 8.6±0.6 | 7.6±0.7 | 16.4±1.3 | 7.4±0.7 | 13.2±1.1 |
| 5d | H | 3-Phenylpropyl | H | CH | 15.3±1.2 | 7.4±0.5 | 11.6±0.7 | 14.6±1.1 | 12.3±0.9 |
| 5e | CH3 | n-Butyl | H | CH | 17.4±1.5 | 9.9±0.8 | 15.8±1.3 | 15.5±1.2 | 17.3±1.4 |
| 5f | CH3 | Benzyl | H | CH | 10.7±0.9 | 17.4±1.5 | 13.1±1.2 | 18.9±1.7 | 14.3±1.2 |
| 5g | CH(CH3)2 | n-Butyl | H | CH | 24.7±2.1 | 14.8±1.2 | 17.2±1.4 | 17.8±1.2 | 16.9±1.5 |
| 5h | CH(CH3)2 | Benzyl | H | CH | 19.2±1.8 | 12.9±1.2 | 15.6±1.4 | 16.3±1.5 | 18.5±1.6 |
| 5i | C6H5 | Benzyl | H | CH | 9.4±0.9 | 12.8±1.2 | 10.2±0.8 | 22.6±1.5 | 13.8±1.1 |
| 5j | C6H4(o-Cl) | n-Butyl | H | CH | 9.5±0.8 | 19.2±1.4 | 17.4±1.2 | 13.4±1.1 | 8.9±0.7 |
| 5k | C6H4(p-OCH3) | Benzyl | H | CH | 13.7±0.8 | 8.9±0.6 | 16.5±1.3 | 10.6±0.9 | 12.4±0.9 |
| 5l | 3-Pyridyl | n-Butyl | H | CH | 15.3±1.2 | 10.1±0.8 | 26.2±1.7 | 15.3±1.2 | 14.9±1.1 |
| 5m | 2-Thienyl | Benzyl | H | CH | 9.8±0.7 | 9.5±0.9 | 12.2±1.1 | 11.5±0.8 | 16.9±1.2 |
| 5n | H | n-Butyl | H | N | 14.7±1.2 | 7.5±0.5 | 18.6±1.1 | 17.8±1.4 | 23.9±1.7 |
| 5o | H | Benzyl | H | N | 21.2±1.9 | 24.7±2.1 | 32.3±2.5 | 21.1±1.8 | 12.4±1.1 |
| 5p | H | n-Butyl | OCH3 | CH | 14.6±1.3 | 24.3±1.9 | 17.3±1.6 | 27.1±1.9 | 25.7±1.6 |
| 5q | H | n-Butyl | CF3 | CH | 8.7±0.7 | 14.8±1.2 | 12.3±0.9 | 9.4±0.7 | 8.6±0.5 |
| 5r | H | Benzyl | OCH3 | CH | 14.5±1.2 | 6.2±0.4 | 19.1±0.7 | 21.4±1.8 | 33.1±2.3 |
| 5s | H | Benzyl | CF3 | CH | 10.1±0.6 | 14.3±0.8 | 18.3±0.5 | 10.1±0.7 | 4.9±0.3 |
| Cisplatin | 15.8±2.4 | 8.4±0.7 | 4.2±0.7 | 15.4±1.9 | 10.5±2.3 | ||||
| Compd. | R1 | R9 | R | X | IC50/(μmol•L–1)±SDa | ||||
|---|---|---|---|---|---|---|---|---|---|
| A549 | BGC-823 | CT-26 | Bel-7402 | MCF-7 | |||||
| 5a | H | n-Butyl | H | CH | 32.1±2.8 | 16.1±1.4 | 17.2±1.3 | 13.3±1.1 | 12.7±0.9 |
| 5b | H | Benzyl | H | CH | 9.7±0.7 | 17.5±1.6 | 13.8±1.1 | 15.6±1.3 | 16.1±1.2 |
| 5c | H | 4-Fluorobenzyl | H | CH | 8.6±0.6 | 7.6±0.7 | 16.4±1.3 | 7.4±0.7 | 13.2±1.1 |
| 5d | H | 3-Phenylpropyl | H | CH | 15.3±1.2 | 7.4±0.5 | 11.6±0.7 | 14.6±1.1 | 12.3±0.9 |
| 5e | CH3 | n-Butyl | H | CH | 17.4±1.5 | 9.9±0.8 | 15.8±1.3 | 15.5±1.2 | 17.3±1.4 |
| 5f | CH3 | Benzyl | H | CH | 10.7±0.9 | 17.4±1.5 | 13.1±1.2 | 18.9±1.7 | 14.3±1.2 |
| 5g | CH(CH3)2 | n-Butyl | H | CH | 24.7±2.1 | 14.8±1.2 | 17.2±1.4 | 17.8±1.2 | 16.9±1.5 |
| 5h | CH(CH3)2 | Benzyl | H | CH | 19.2±1.8 | 12.9±1.2 | 15.6±1.4 | 16.3±1.5 | 18.5±1.6 |
| 5i | C6H5 | Benzyl | H | CH | 9.4±0.9 | 12.8±1.2 | 10.2±0.8 | 22.6±1.5 | 13.8±1.1 |
| 5j | C6H4(o-Cl) | n-Butyl | H | CH | 9.5±0.8 | 19.2±1.4 | 17.4±1.2 | 13.4±1.1 | 8.9±0.7 |
| 5k | C6H4(p-OCH3) | Benzyl | H | CH | 13.7±0.8 | 8.9±0.6 | 16.5±1.3 | 10.6±0.9 | 12.4±0.9 |
| 5l | 3-Pyridyl | n-Butyl | H | CH | 15.3±1.2 | 10.1±0.8 | 26.2±1.7 | 15.3±1.2 | 14.9±1.1 |
| 5m | 2-Thienyl | Benzyl | H | CH | 9.8±0.7 | 9.5±0.9 | 12.2±1.1 | 11.5±0.8 | 16.9±1.2 |
| 5n | H | n-Butyl | H | N | 14.7±1.2 | 7.5±0.5 | 18.6±1.1 | 17.8±1.4 | 23.9±1.7 |
| 5o | H | Benzyl | H | N | 21.2±1.9 | 24.7±2.1 | 32.3±2.5 | 21.1±1.8 | 12.4±1.1 |
| 5p | H | n-Butyl | OCH3 | CH | 14.6±1.3 | 24.3±1.9 | 17.3±1.6 | 27.1±1.9 | 25.7±1.6 |
| 5q | H | n-Butyl | CF3 | CH | 8.7±0.7 | 14.8±1.2 | 12.3±0.9 | 9.4±0.7 | 8.6±0.5 |
| 5r | H | Benzyl | OCH3 | CH | 14.5±1.2 | 6.2±0.4 | 19.1±0.7 | 21.4±1.8 | 33.1±2.3 |
| 5s | H | Benzyl | CF3 | CH | 10.1±0.6 | 14.3±0.8 | 18.3±0.5 | 10.1±0.7 | 4.9±0.3 |
| Cisplatin | 15.8±2.4 | 8.4±0.7 | 4.2±0.7 | 15.4±1.9 | 10.5±2.3 | ||||

| [1] |
(a) https://www.cancer.gov/about-cancer/understanding/statistics;
|
|
(b) http://www.who.int/mediacentre/factsheets/fs297/en.
|
|
| [2] |
Siegel, R. L.; Miller, K. D.; Jemal, A. C. A. Cancer J. Clin. 2017, 67, 7.
doi: 10.3322/caac.21387 |
| [3] |
Yan, L.; Lin, M.; Pan, S.; Assaraf, Y. G.; Wang, Z. W.; Zhu, X. Drug Resistance Updates 2020, 49, 100673.
doi: 10.1016/j.drup.2019.100673 |
| [4] |
Chatterjee, N.; Bivona, T. G. Trends Cancer 2019, 5, 170.
doi: S2405-8033(19)30019-6 pmid: 30898264 |
| [5] |
Gaujac, A.; Navickiene, S.; Collins, M. I.; Brandt, S. D.; Andrade, J. B. Drug Test. Anal. 2012, 4, 636.
doi: 10.1002/dta.1343 pmid: 22577086 |
| [6] |
Xie, Z. J.; Cao, N.; Wang, C. H. Food Chem. 2021, 348, 129067.
doi: 10.1016/j.foodchem.2021.129067 |
| [7] |
Cao, R. H.; Peng, W. L.; Wang, Z. H.; Xu, A. L. Curr. Med. Chem. 2007, 14, 497.
|
| [8] |
Michael, C.; Robert, W. W.; Fil, G.; James, M. C.; Steven, A. B.; Kenner, C. R.; Jacqueline, N. C.; Steven, M. P., Phil, S. J. Med. Chem. 1982, 25, 1081.
doi: 10.1021/jm00351a015 |
| [9] |
Bournine, L.; Bensalem, S.; Fatmi, S.; Bedjou, F.; Mathieu, V.; Iguer-Ouada, M.; Kiss, R.; Duez, P. Eur. J. Integr. Med. 2017, 9, 91.
doi: 10.1016/j.eujim.2016.10.002 |
| [10] |
Sun, Y.; Guo, L.; Fan, W.-X.; Chen, W.; Zhang, J.; Dai, B. Chin. J. Org. Chem. 2021, 41, 400. (in Chinese)
doi: 10.6023/cjoc202006026 |
|
( 孙跃, 郭亮, 范文玺, 陈伟, 张洁, 代斌, 有机化学, 2021, 41, 400.)
doi: 10.6023/cjoc202006026 |
|
| [11] |
Chen, X. F.; Guo, L.; Ma, Q.; Chen, W.; Fan, W. X.; Zhang, J. Molecules 2019, 24, 2950.
doi: 10.3390/molecules24162950 |
| [12] |
Kamboj, A.; Sihag, B.; Brar, D. S.; Kaur, A.; Salunke, D. B. Eur. J. Med. Chem. 2021, 221, 113536.
doi: 10.1016/j.ejmech.2021.113536 |
| [13] |
Srivastava, S. K.; Agarwal, A.; Chauhan, P. M. S.; Agarwal, S. K.; Bhaduri, A. P.; Singh, S. N.; Fatima, N.; Chatterjee, R. K. P. Bioorg. Med. Chem. 1999, 7, 1223.
doi: 10.1016/S0968-0896(99)00050-4 |
| [14] |
Wang, Y. H.; Tang, J. G.; Wang, R. R.; Yang, L. M.; Dong, Z. J.; Du, L.; Shen, X.; Liu, J. K.; Zheng, Y. T. Biochem. Biophys. Res. Commun. 2007, 355, 1091.
doi: 10.1016/j.bbrc.2007.02.081 |
| [15] |
Guo, L.; Xie, J. W.; Fan, W. X.; Chen, W.; Dai, B.; Ma, Q. Chin. J. Org. Chem. 2017, 37, 1741. (in Chinese)
doi: 10.6023/cjoc201701005 |
|
( 郭亮, 谢建伟, 范文玺, 陈伟, 代斌, 马芹, 有机化学, 2017, 37, 1741.)
doi: 10.6023/cjoc201701005 |
|
| [16] |
Sun, Y.; Huo, X.-Y.; Wang, Z.-X.; Han, X.-Q.; Zhang, J. Fine Chem. 2020, 37, 1672. (in Chinese)
|
|
( 孙跃, 霍新玉, 王兆旭, 韩小强, 张洁, 精细化工, 2020, 37, 1672.)
|
|
| [17] |
Huo, X. Y.; Li, W. B.; Zhang, B. Y.; Chen, X. F.; Dai, B. Chin. J. Org. Chem. 2018, 38, 3356. (in Chinese)
doi: 10.6023/cjoc201805053 |
|
( 霍新玉, 李文斌, 张博雅, 陈晓飞, 代斌, 有机化学, 2018, 38, 3356.)
doi: 10.6023/cjoc201805053 |
|
| [18] |
Guo, L.; Chen, W.; Fan, W. X.; Ma, Q.; Sun, R. Q.; Shao, G.; Cao, R. H. Med. Chem. Commun. 2016, 7, 2177.
doi: 10.1039/C6MD00360E |
| [19] |
Huo, X. Y.; Guo, L.; Chen, X. F.; Zhou, Y. T.; Zhang, J.; Han, X. Q.; Dai, B. Molecules 2018, 23, 1344.
doi: 10.3390/molecules23061344 |
| [20] |
Narasimhan, B.; Sharma, D.; Kumar, P. Med Chem Res. 2012, 21, 269.
doi: 10.1007/s00044-010-9533-9 |
| [21] |
Shah, K.; Chhabra, S.; Shrivastava, S. K.; Mishra, P. Med. Chem. Res. 2013, 22, 5077.
doi: 10.1007/s00044-013-0476-9 |
| [22] |
Gaba, M.; Singh, S.; Mohan, C. Eur. J. Med. Chem. 2014, 76, 494.
doi: 10.1016/j.ejmech.2014.01.030 |
| [23] |
Li, N.; Xin, J.-C.; Meng, Y.-Q.; Li, E.-D.; Ma, Q.-S.; Bao, C.-N. Chin. J. Org. Chem. 2018, 38, 368. (in Chinese)
|
|
( 栗娜, 辛景超, 孟娅琪, 李二冬, 马启胜, 包崇男, 有机化学, 2018, 38, 368.)
|
|
| [24] |
Hsieh, C. Y.; Ko, P. W.; Chang, Y. J.; Kapoor, M.; Liang, Y. C.; Chu, H. L. Molecules 2019, 24, 3299.
doi: 10.3390/molecules24183299 |
| [25] |
Abonia, R.; Cortés, E.; Insuasty., B.; Insuasty, B.; Quiroga, J.; Nogueras, M.; Coboet, J. Eur. J. Med. Chem. 2011, 46, 4062.
doi: 10.1016/j.ejmech.2011.06.006 |
| [26] |
Gaba, M.; Singh, S.; Mohan, C. Eur. J. Med. Chem. 2014, 76, 494.
doi: 10.1016/j.ejmech.2014.01.030 |
| [27] |
(a) Sharma, D.; Narasimhan, B.; Kumar, P.; Judge, V.; Narang, R.; Clercq, E. D. J. Enzyme Inhib. Med. Chem. 2009, 24, 1161.
doi: 10.1080/14756360802694427 |
|
(b) Chidambaranathan, V.; Mahalakshmi, C. M. Int. J. Chem. Sci. 2015, 13, 205.
|
|
| [28] |
Karaburun, A. C.; Cavusoglu, K. C.; Cevik, U. A.; Osmaniye, D.; Sağlık, B. N.; Levent, S.; Atlı, O.; Koparal, A. S.; Kaplancıklı, Z. A. Molecules 2019, 24, 191.
doi: 10.3390/molecules24010191 |
| [29] |
Sridevi, C. H.; Balaji, K.; Naidu, A.; Sudhakaran, R. Eur. J. Inorg. Chem. 2012, 7, 234.
|
| [30] |
Chen, Z. Y.; Cao, R. H.; Shi, B. X.; Guo, L.; Sun, J.; Ma, Q.; Fan, W. X.; Song, H. C. Eur. J. Med. Chem. 2011, 46, 5127.
doi: 10.1016/j.ejmech.2011.08.027 |
| [31] |
He, S.; Dobbelaar, P. H.; Guo, L.; Ye, Z.; Jian, L.; Jian, T. Biochem. Biophys. Res. Commun. 2016, 26, 1529.
|
| [32] |
Chen, Q.; Chen, W.; Fan, W. X.; Guo, L.; Ma, Q.; Zhang, X.D.; Du, R. L.; Cao, R. H. Bioorg. Med. Chem. Lett. 2016, 26, 5065.
doi: 10.1016/j.bmcl.2016.08.084 |
| [33] |
Zhao, Y. X.; Wang, Y. Y.; Zhang, C. L.; Xu, X.; Wang, S. F. Chin. J. Org. Chem. 2021, 41, 1224. (in Chinese)
doi: 10.6023/cjoc202009050 |
|
( 赵雨珣, 王芸芸, 张成龙, 徐徐, 王石发, 有机化学, 2021, 41, 1224.)
doi: 10.6023/cjoc202009050 |
| [1] | 王博珍, 张婕, 粘春惠, 金茗茗, 孔苗苗, 李物兰, 何文斐, 吴建章. 含有3,4-二氯苯基的酰胺类化合物的合成及抗肿瘤活性研究[J]. 有机化学, 2024, 44(1): 232-241. |
| [2] | 霍海波, 李桂霞, 王世军, 韩春, 师宝君, 李健. 新型γ-咔啉衍生物的合成及其抑菌活性研究[J]. 有机化学, 2024, 44(1): 204-215. |
| [3] | 肖梦佳, 高希珂. 含苯并咪唑结构的薁类衍生物的设计合成及抗炎活性研究[J]. 有机化学, 2023, 43(9): 3246-3256. |
| [4] | 曹瑞霞, 贾玉萍. 含香豆素的吡咯并[2,3-d]嘧啶衍生物的合成及生物活性研究[J]. 有机化学, 2023, 43(9): 3304-3311. |
| [5] | 张维舒, 聂礼飞, Khurshed Bozorov, 阿吉艾克拜尔•艾萨, 赵江瑜. 2,5-二氨基噻吩-3,4-二羧酸二乙酯衍生物的合成及抗肿瘤活性研究[J]. 有机化学, 2023, 43(7): 2543-2552. |
| [6] | 左鑫, 许诗诺, 陈忠洋, 鄢剑锋, 袁耀锋. 茂铁类单分子结电子传输性质的研究进展[J]. 有机化学, 2023, 43(7): 2313-2322. |
| [7] | 何金燕, 田富云, 吴青青, 郑月明, 陈玉婷, 许海燕, 金正盛, 詹丽, 程新强, 顾跃玲, 高召兵, 赵桂龙. 基于[3.3.3]螺桨烷的电压门控钙离子通道α2δ亚基配体的合成和生物活性研究[J]. 有机化学, 2023, 43(6): 2226-2238. |
| [8] | 庞盼杏, 宁蓉, 祝创, 黄文洁, 马献力, 蒋彩娜, 李芳耀, 周小群. 苦参碱缩氨基脲类化合物的合成及其体外抗肿瘤活性研究[J]. 有机化学, 2023, 43(6): 2126-2135. |
| [9] | 钟玉梅, 邹小颖, 卓小丫, 王逸涵, 申佳奕, 郑绿茵, 郭维. 4-氧代-2-亚胺基噻唑烷-5-亚基乙酸乙酯类化合物的设计、合成及抗癌活性[J]. 有机化学, 2023, 43(4): 1452-1461. |
| [10] | 刘兴周, 于明加, 梁建华. 原小檗碱骨架的合成及其抗炎活性研究进展[J]. 有机化学, 2023, 43(4): 1325-1340. |
| [11] | 段康慧, 唐俊龙, 伍婉卿. 稠杂环化合物的合成及其抗肿瘤活性研究进展[J]. 有机化学, 2023, 43(3): 826-854. |
| [12] | 廖楚婕, 阮洪瑶, 姜峻峰, 罗伦, 胡扬根. 3-芳基-2-亚胺-苯并[e]-1,3-噁嗪-4-醇衍生物的合成及活性评价[J]. 有机化学, 2023, 43(2): 763-770. |
| [13] | 张雨杉, 桓臻, 杨金东, 程津培. 氮杂环磷氢试剂的氢转移活性研究进展[J]. 有机化学, 2023, 43(11): 3806-3825. |
| [14] | 张炳文, 林雨琦, 薛彦青, 王婧, 杨文超, 王晓峰, 刘文. 禾谷镰刀菌次级代谢产物研究[J]. 有机化学, 2023, 43(11): 4003-4007. |
| [15] | 刘威琴, 邵利辉, 李成朋, 邹雅玉, 龙海洮, 李焱, 戈强胜, 王贞超, 欧阳贵平. 3-腙喹唑啉酮衍生物的合成及抗肿瘤活性研究[J]. 有机化学, 2023, 43(1): 214-222. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||