| [1] Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2008, 108, 5299.
[2] Chen, H.; Han, J.; Wang L. Angew. Chem., Int. Ed. 2018, 57, 12313.
[3] Zhang, Y.; Han, J.; Liu, Z.-J. RSC Adv. 2015, 5, 25485.
[4] Kuik, W.-J.; Kema, I. P.; Brouwers, A. H.; Zijlma, R.; Neumann, K. D.; Dierckx, R. A. J. O.; DiMagno, S. G.; Elsinga, P. H. J. Nucl. Med. 2015, 56, 106.
[5] DeSolms, S. J.; Woltersdorf, O. W., Jr.; Cragoe, E. J., Jr.; Watson, L. S.; Fanelli, G. M., Jr. J. Med. Chem. 1978, 215, 437.
[6] Das, P.; Etsuko T.; Tokunaga, H.; Akiyama, H.; Doi, H.; Saito, N.; Shibata, N. Beilstein J. Org. Chem. 2018, 14, 364.
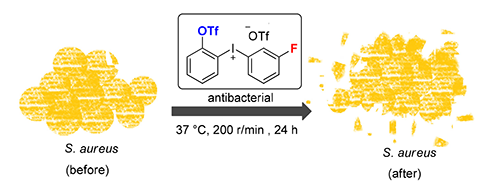
[7] Goldstein, E. J. C.; Citron, D. M.; Warren, Y.; Merriam, C. V.; Tyrrell, K.; Fernandez, H.; Radhakrishnan, U.; Stang, P. J.; Conrads, G. Antimicrob. Agents Chemother. 2004, 48, 2766.
[8] Lu, J.; Risbood, P.; Kane, C. T., Jr.; Hossain, M. T.; Anderson, L.; Hill, K.; Monks, A.; Wu, Y.; Antony, S.; Juhasz, A.; Liu, H.; Jiang, G.; Harris, E.; Roy, K.; Meitzler, J. L.; Konaté, M.; Doroshow, J. H. Biochem. Pharmacol. 2017, 143, 25.
[9] Hui, F.; Debiemme-Chouvy, C. Biomacromolecules 2013, 14, 585.
[10] Dong, A.; Wang, Y.-J.; Gao, Y.; Gao, T.; Gao G. Chem. Rev. 2017, 117, 4806.
[11] Gottardi, W.; Debabov, D.; Nagl, M. Antimicrob. Agents Chemother. 2013, 57, 1107.
[12] Fang, G.; Li, W.; Shen, X.; Perez-Aguilar, J. M.; Chong, Y.; Gao, X..; Chai, Z.; Chen, C.; Ge, C.; Zhou, R. Nat. Commun. 2018, 9, 129.
[13] Hoque, J.; Akkapeddi, P.; Yarlagadda, V.; Uppu. D. S. S. M.; Kumar, P.; Haldar, J. Langmuir 2012, 28, 12225.
[14] Li, Y.-K.; Liu, B.; Zhang, L.-J.; Zhu, J.-H.; Wan, C.-P.; Mao, Z.-W. Chin. J. Org. Chem. 2018, 38, 949(in Chinese). (黎勇坤, 刘蓓, 张丽君, 朱加洪, 万春平, 毛泽伟, 有机化学, 2018, 38, 949.) |