有机化学 ›› 2021, Vol. 41 ›› Issue (2): 738-756.DOI: 10.6023/cjoc202007068 上一篇 下一篇
研究论文
石军1, 罗娜1, 丁慕晗1, 李传会1, 万苏然1, 李培甲1, 李君荭1, 鲍小平1,*( )
)
收稿日期:2020-07-29
修回日期:2020-08-11
发布日期:2020-09-16
通讯作者:
鲍小平
作者简介:基金资助:
Jun Shi1, Na Luo1, Muhan Ding1, Chuanhui Li1, Suran Wan1, Peijia Li1, Junhong Li1, Xiaoping Bao1,*( )
)
Received:2020-07-29
Revised:2020-08-11
Published:2020-09-16
Contact:
Xiaoping Bao
Supported by:文章分享

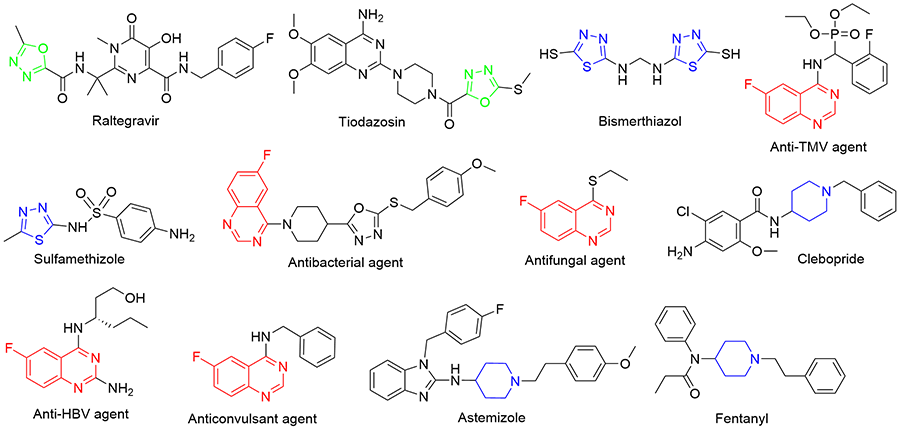
为寻找高效农用抗菌先导化合物, 通过活性亚结构拼接法设计合成了50个含6-氟喹唑啉片段的新型1,3,4-噁(噻)二唑类衍生物6a~6y和8a~8y, 其结构经1H NMR、13C NMR和HRMS手段进行了表征, 且化合物6i和8x的结构最终由X射线单晶衍射法加以确认. 初步抗菌测试表明, 部分化合物表现出较好的体外抗真菌活性. 在50 μg/mL浓度下, 化合物 6b、6d、6t、6v和6x对小麦赤霉病菌的抑制率分别为58%、58%、55%、63%和60%, 化合物6v和8v对苹果腐烂病菌的抑制率分别为71%和64%, 化合物6v对油菜炭疽病菌的抑制率为72%, 它们均优于对照药剂噁霉灵(分别为51%、61%和70%). 此外, 部分化合物在100 μg/mL浓度下也表现出一定的体外抗细菌活性.
石军, 罗娜, 丁慕晗, 李传会, 万苏然, 李培甲, 李君荭, 鲍小平. 含6-氟喹唑啉片段的新型1,3,4-噁(噻)二唑类衍生物的合成及抗菌活性研究[J]. 有机化学, 2021, 41(2): 738-756.
Jun Shi, Na Luo, Muhan Ding, Chuanhui Li, Suran Wan, Peijia Li, Junhong Li, Xiaoping Bao. Synthesis and Antimicrobial Activities of Novel 1,3,4-Oxa(Thia)- diazole Derivatives Containing 6-Fluoroquinazoline Moiety[J]. Chinese Journal of Organic Chemistry, 2021, 41(2): 738-756.
| Compd. | Inhibition ratea/% | Compd. | Inhibition ratea/% | ||||
|---|---|---|---|---|---|---|---|
| GZc | CGc | CMc | GZc | CGc | CMc | ||
| 6a | 40±2 | 20±1 | 17±2 | 8a | 45±1 | 47±1 | 50±1 |
| 6b | 58±2 | 42±0 | 45±0 | 8b | 31 ± 1 | 34±1 | 40±1 |
| 6c | 11±0 | 16±2 | 15±3 | 8c | 30 ± 2 | 32±2 | 33±2 |
| 6d | 58±2 | 52±1 | 55±2 | 8d | 26±1 | 29±2 | 33±2 |
| 6e | 40±2 | 13±1 | 15±3 | 8e | 32±1 | 7±1 | 18±2 |
| 6f | 43±2 | 11±1 | 18±1 | 8f | 25±1 | 16±2 | 18±2 |
| 6g | 22±1 | 14±2 | 16±0 | 8g | 19±1 | 32±1 | 34±2 |
| 6h | 37±2 | 13±1 | 17±3 | 8h | 28±1 | 23±3 | 22±3 |
| 6i | 52±1 | 29±2 | 30±3 | 8i | 30±1 | 19±3 | 18±1 |
| 6j | 9±1 | 22±3 | 13±2 | 8j | 28±1 | 28±2 | 27±1 |
| 6k | 34±3 | 22±1 | 25±2 | 8k | 0 | 9±2 | 9±2 |
| 6l | 38±1 | 26±3 | 26±2 | 8l | 21±1 | 23±3 | 21±2 |
| 6m | 0 | 0 | 8±3 | 8m | 13±3 | 21±1 | 17±1 |
| 6n | 43±3 | 24±1 | 25±2 | 8n | 16±1 | 15±3 | 22±3 |
| 6o | 37±2 | 13±2 | 8±1 | 8o | 0 | 15±1 | 22±3 |
| 6p | 31±1 | 6±2 | 10±2 | 8p | 0 | 0 | 0 |
| 6q | 43±1 | 25±2 | 29±3 | 8q | 31±1 | 7±2 | 10±1 |
| 6r | 34±1 | 0 | 9±3 | 8r | 12±1 | 16±3 | 15±3 |
| 6s | 43±1 | 21±0 | 26±1 | 8s | 20±1 | 7±2 | 9±2 |
| 6t | 55±3 | 43±2 | 47±1 | 8t | 46±1 | 36±2 | 44±1 |
| 6u | 51±2 | 21±1 | 19±2 | 8u | 11±1 | 21±2 | 25±2 |
| 6v | 63±1 | 72±1 | 71±1 | 8v | 54±1 | 50±2 | 64±2 |
| 6w | 16±2 | 13±2 | 13±2 | 8w | 17±3 | 22±2 | 14±3 |
| 6x | 60±2 | 33±2 | 45±0 | 8x | 16±1 | 15±2 | 21±3 |
| 6y | 16±3 | 11±2 | 31±4 | 8y | 26±1 | 23±2 | 24±2 |
| Hymexazolb | 51±1 | 70±1 | 61±1 | ||||
| Compd. | Inhibition ratea/% | Compd. | Inhibition ratea/% | ||||
|---|---|---|---|---|---|---|---|
| GZc | CGc | CMc | GZc | CGc | CMc | ||
| 6a | 40±2 | 20±1 | 17±2 | 8a | 45±1 | 47±1 | 50±1 |
| 6b | 58±2 | 42±0 | 45±0 | 8b | 31 ± 1 | 34±1 | 40±1 |
| 6c | 11±0 | 16±2 | 15±3 | 8c | 30 ± 2 | 32±2 | 33±2 |
| 6d | 58±2 | 52±1 | 55±2 | 8d | 26±1 | 29±2 | 33±2 |
| 6e | 40±2 | 13±1 | 15±3 | 8e | 32±1 | 7±1 | 18±2 |
| 6f | 43±2 | 11±1 | 18±1 | 8f | 25±1 | 16±2 | 18±2 |
| 6g | 22±1 | 14±2 | 16±0 | 8g | 19±1 | 32±1 | 34±2 |
| 6h | 37±2 | 13±1 | 17±3 | 8h | 28±1 | 23±3 | 22±3 |
| 6i | 52±1 | 29±2 | 30±3 | 8i | 30±1 | 19±3 | 18±1 |
| 6j | 9±1 | 22±3 | 13±2 | 8j | 28±1 | 28±2 | 27±1 |
| 6k | 34±3 | 22±1 | 25±2 | 8k | 0 | 9±2 | 9±2 |
| 6l | 38±1 | 26±3 | 26±2 | 8l | 21±1 | 23±3 | 21±2 |
| 6m | 0 | 0 | 8±3 | 8m | 13±3 | 21±1 | 17±1 |
| 6n | 43±3 | 24±1 | 25±2 | 8n | 16±1 | 15±3 | 22±3 |
| 6o | 37±2 | 13±2 | 8±1 | 8o | 0 | 15±1 | 22±3 |
| 6p | 31±1 | 6±2 | 10±2 | 8p | 0 | 0 | 0 |
| 6q | 43±1 | 25±2 | 29±3 | 8q | 31±1 | 7±2 | 10±1 |
| 6r | 34±1 | 0 | 9±3 | 8r | 12±1 | 16±3 | 15±3 |
| 6s | 43±1 | 21±0 | 26±1 | 8s | 20±1 | 7±2 | 9±2 |
| 6t | 55±3 | 43±2 | 47±1 | 8t | 46±1 | 36±2 | 44±1 |
| 6u | 51±2 | 21±1 | 19±2 | 8u | 11±1 | 21±2 | 25±2 |
| 6v | 63±1 | 72±1 | 71±1 | 8v | 54±1 | 50±2 | 64±2 |
| 6w | 16±2 | 13±2 | 13±2 | 8w | 17±3 | 22±2 | 14±3 |
| 6x | 60±2 | 33±2 | 45±0 | 8x | 16±1 | 15±2 | 21±3 |
| 6y | 16±3 | 11±2 | 31±4 | 8y | 26±1 | 23±2 | 24±2 |
| Hymexazolb | 51±1 | 70±1 | 61±1 | ||||
| Compd. | Xanthomonas axonopodis pv. citri | Xanthomonas oryzae pv. oryzae | Ralstonia solanacearum | |||||
|---|---|---|---|---|---|---|---|---|
| 100 μg/mL | 50 μg/mL | 100 μg/mL | 50 μg/mL | 100 μg/mL | 50 μg/mL | |||
| 6a | 38±4 | 33±2 | 32±3 | 18±2 | 9±3 | 5±3 | ||
| 6b | 32±3 | 16±3 | 27±3 | 22±5 | 21±2 | 14±3 | ||
| 6c | 40±3 | 30±2 | 43±3 | 36±1 | 20±5 | 13±4 | ||
| 6d | 38±3 | 28±2 | 31±3 | 30±2 | 14±3 | 12±5 | ||
| 6e | 31±4 | 27±4 | 29±3 | 15±0 | 18±2 | 17±1 | ||
| 6f | 41±4 | 25±5 | 27±4 | 24±4 | 17±1 | 6±3 | ||
| 6g | 39±3 | 20±2 | 35±1 | 17±3 | 21±4 | 16±2 | ||
| 6h | 39±4 | 29±2 | 27±1 | 21±4 | 28±0 | 17±4 | ||
| 6i | 32±2 | 30±4 | 33±6 | 23±5 | 33±2 | 24±3 | ||
| 6j | 31±5 | 26±5 | 37±3 | 14±2 | 18±3 | 15±2 | ||
| 6k | 35±5 | 24±4 | 18±5 | 13±2 | 27±3 | 13±4 | ||
| 6l | 38±3 | 27±3 | 26±3 | 19±3 | 47±2 | 23±3 | ||
| 6m | 35±3 | 20±5 | 32±5 | 29±2 | 17±2 | 16±2 | ||
| 6n | 36±3 | 26±5 | 33±3 | 21±3 | 25±2 | 20±3 | ||
| 6o | 38±1 | 27±3 | 26±2 | 11±4 | 29±3 | 9±4 | ||
| 6p | 39±1 | 30±3 | 20±4 | 17±4 | 19±2 | 13±5 | ||
| 6q | 31±6 | 21±2 | 16±5 | 7±1 | 15±4 | 9±3 | ||
| 6r | 41±3 | 28±3 | 21±3 | 14±1 | 26±2 | 21±3 | ||
| 6s | 50±4 | 37±2 | 33±2 | 30±5 | 17±3 | 13±3 | ||
| 6t | 45±3 | 32±3 | 37±5 | 20±3 | 26±5 | 18±3 | ||
| 6u | 40±2 | 30±2 | 40±4 | 36±7 | 33±3 | 29±3 | ||
| 6v | 44±4 | 34±2 | 33±1 | 18±1 | 34±3 | 31±5 | ||
| 6w | 37±4 | 28±5 | 45±5 | 27±2 | 35±3 | 33±3 | ||
| 6x | 41±3 | 33±3 | 49±4 | 22±5 | 30±2 | 16±2 | ||
| 6y | 29±4 | 21±4 | 57±2 | 47±2 | 48±4 | 35±3 | ||
| 8a | 44±5 | 27±3 | 30±6 | 27±3 | 25±4 | 23±3 | ||
| 8b | 54±3 | 32±2 | 32±4 | 22±2 | 34±3 | 28±3 | ||
| 8c | 42±1 | 35±4 | 40±1 | 35±4 | 22±5 | 18±4 | ||
| 8d | 41±4 | 32±4 | 37±1 | 32±4 | 44±2 | 37±4 | ||
| 8e | 32±0 | 29±3 | 35±2 | 29±3 | 48±2 | 37±1 | ||
| 8f | 38±2 | 29±3 | 33±5 | 29±3 | 49±2 | 41±3 | ||
| 8g | 31±1 | 26±4 | 30±8 | 26±4 | 36±5 | 30±4 | ||
| 8h | 36±4 | 29±1 | 34±3 | 29±1 | 20±4 | 17±4 | ||
| 8i | 36±2 | 31±5 | 27±6 | 31±5 | 28±3 | 24±1 | ||
| 8j | 40±3 | 38±3 | 38±8 | 35±3 | 66±1 | 46±1 | ||
| 8k | 33±1 | 30±1 | 39±3 | 32±1 | 29±4 | 21±4 | ||
| 8l | 43±2 | 31±1 | 32±8 | 31±1 | 35±3 | 33±2 | ||
| 8m | 40±4 | 28±1 | 34±4 | 28±1 | 24±3 | 23±3 | ||
| 8n | 33±3 | 30±2 | 37±3 | 30±2 | 17±4 | 12±6 | ||
| 8o | 29±2 | 20±1 | 23±8 | 20±1 | 22±3 | 13±3 | ||
| 8p | 39±4 | 28±1 | 35±8 | 28±1 | 21±4 | 15±4 | ||
| 8q | 43±4 | 30±4 | 41±0 | 30±4 | 33±3 | 31±2 | ||
| 8r | 43±3 | 31±3 | 34±5 | 31±3 | 40±4 | 35±4 | ||
| 8s | 42±3 | 39±4 | 40±2 | 32±4 | 30±2 | 23±7 | ||
| 8t | 40±4 | 34±2 | 40±3 | 34±2 | 34±2 | 33±5 | ||
| 8u | 39±5 | 31±5 | 36±4 | 31±5 | 30±3 | 27±3 | ||
| 8v | 44±3 | 25±2 | 31±3 | 20±1 | 28±2 | 16±4 | ||
| 8w | 36±2 | 29±2 | 26±3 | 21±3 | 24±4 | 14±2 | ||
| 8x | 56±3 | 52±2 | 52±3 | 40±2 | 56±3 | 37±4 | ||
| 8y | 47±5 | 35±2 | 40±4 | 35±2 | 32±3 | 21±1 | ||
| BMTb | 54±1 | 37±4 | 60±2 | 43±5 | 50±4 | 33±2 | ||
| TDCb | NTc | NTc | NTc | NTc | 43±3 | 27±2 | ||
| Compd. | Xanthomonas axonopodis pv. citri | Xanthomonas oryzae pv. oryzae | Ralstonia solanacearum | |||||
|---|---|---|---|---|---|---|---|---|
| 100 μg/mL | 50 μg/mL | 100 μg/mL | 50 μg/mL | 100 μg/mL | 50 μg/mL | |||
| 6a | 38±4 | 33±2 | 32±3 | 18±2 | 9±3 | 5±3 | ||
| 6b | 32±3 | 16±3 | 27±3 | 22±5 | 21±2 | 14±3 | ||
| 6c | 40±3 | 30±2 | 43±3 | 36±1 | 20±5 | 13±4 | ||
| 6d | 38±3 | 28±2 | 31±3 | 30±2 | 14±3 | 12±5 | ||
| 6e | 31±4 | 27±4 | 29±3 | 15±0 | 18±2 | 17±1 | ||
| 6f | 41±4 | 25±5 | 27±4 | 24±4 | 17±1 | 6±3 | ||
| 6g | 39±3 | 20±2 | 35±1 | 17±3 | 21±4 | 16±2 | ||
| 6h | 39±4 | 29±2 | 27±1 | 21±4 | 28±0 | 17±4 | ||
| 6i | 32±2 | 30±4 | 33±6 | 23±5 | 33±2 | 24±3 | ||
| 6j | 31±5 | 26±5 | 37±3 | 14±2 | 18±3 | 15±2 | ||
| 6k | 35±5 | 24±4 | 18±5 | 13±2 | 27±3 | 13±4 | ||
| 6l | 38±3 | 27±3 | 26±3 | 19±3 | 47±2 | 23±3 | ||
| 6m | 35±3 | 20±5 | 32±5 | 29±2 | 17±2 | 16±2 | ||
| 6n | 36±3 | 26±5 | 33±3 | 21±3 | 25±2 | 20±3 | ||
| 6o | 38±1 | 27±3 | 26±2 | 11±4 | 29±3 | 9±4 | ||
| 6p | 39±1 | 30±3 | 20±4 | 17±4 | 19±2 | 13±5 | ||
| 6q | 31±6 | 21±2 | 16±5 | 7±1 | 15±4 | 9±3 | ||
| 6r | 41±3 | 28±3 | 21±3 | 14±1 | 26±2 | 21±3 | ||
| 6s | 50±4 | 37±2 | 33±2 | 30±5 | 17±3 | 13±3 | ||
| 6t | 45±3 | 32±3 | 37±5 | 20±3 | 26±5 | 18±3 | ||
| 6u | 40±2 | 30±2 | 40±4 | 36±7 | 33±3 | 29±3 | ||
| 6v | 44±4 | 34±2 | 33±1 | 18±1 | 34±3 | 31±5 | ||
| 6w | 37±4 | 28±5 | 45±5 | 27±2 | 35±3 | 33±3 | ||
| 6x | 41±3 | 33±3 | 49±4 | 22±5 | 30±2 | 16±2 | ||
| 6y | 29±4 | 21±4 | 57±2 | 47±2 | 48±4 | 35±3 | ||
| 8a | 44±5 | 27±3 | 30±6 | 27±3 | 25±4 | 23±3 | ||
| 8b | 54±3 | 32±2 | 32±4 | 22±2 | 34±3 | 28±3 | ||
| 8c | 42±1 | 35±4 | 40±1 | 35±4 | 22±5 | 18±4 | ||
| 8d | 41±4 | 32±4 | 37±1 | 32±4 | 44±2 | 37±4 | ||
| 8e | 32±0 | 29±3 | 35±2 | 29±3 | 48±2 | 37±1 | ||
| 8f | 38±2 | 29±3 | 33±5 | 29±3 | 49±2 | 41±3 | ||
| 8g | 31±1 | 26±4 | 30±8 | 26±4 | 36±5 | 30±4 | ||
| 8h | 36±4 | 29±1 | 34±3 | 29±1 | 20±4 | 17±4 | ||
| 8i | 36±2 | 31±5 | 27±6 | 31±5 | 28±3 | 24±1 | ||
| 8j | 40±3 | 38±3 | 38±8 | 35±3 | 66±1 | 46±1 | ||
| 8k | 33±1 | 30±1 | 39±3 | 32±1 | 29±4 | 21±4 | ||
| 8l | 43±2 | 31±1 | 32±8 | 31±1 | 35±3 | 33±2 | ||
| 8m | 40±4 | 28±1 | 34±4 | 28±1 | 24±3 | 23±3 | ||
| 8n | 33±3 | 30±2 | 37±3 | 30±2 | 17±4 | 12±6 | ||
| 8o | 29±2 | 20±1 | 23±8 | 20±1 | 22±3 | 13±3 | ||
| 8p | 39±4 | 28±1 | 35±8 | 28±1 | 21±4 | 15±4 | ||
| 8q | 43±4 | 30±4 | 41±0 | 30±4 | 33±3 | 31±2 | ||
| 8r | 43±3 | 31±3 | 34±5 | 31±3 | 40±4 | 35±4 | ||
| 8s | 42±3 | 39±4 | 40±2 | 32±4 | 30±2 | 23±7 | ||
| 8t | 40±4 | 34±2 | 40±3 | 34±2 | 34±2 | 33±5 | ||
| 8u | 39±5 | 31±5 | 36±4 | 31±5 | 30±3 | 27±3 | ||
| 8v | 44±3 | 25±2 | 31±3 | 20±1 | 28±2 | 16±4 | ||
| 8w | 36±2 | 29±2 | 26±3 | 21±3 | 24±4 | 14±2 | ||
| 8x | 56±3 | 52±2 | 52±3 | 40±2 | 56±3 | 37±4 | ||
| 8y | 47±5 | 35±2 | 40±4 | 35±2 | 32±3 | 21±1 | ||
| BMTb | 54±1 | 37±4 | 60±2 | 43±5 | 50±4 | 33±2 | ||
| TDCb | NTc | NTc | NTc | NTc | 43±3 | 27±2 | ||
| [1] |
Li D.; Zhang S.; Song Z.; Wang G.; Li S. Eur. J. Med. Chem. 2017, 136, 114.
doi: 10.1016/j.ejmech.2017.04.073 |
| [2] |
Wang L.; Li C.; Zhang Y.; Qiao C.; Ye Y. J. Agric. Food Chem. 2013, 61, 8632.
doi: 10.1021/jf402388x |
| [3] |
Price C.L.; Parker J.E.; Warrilow A. G. S.; Kelly D.E.; Kelly S.L. Pest Manage. Sci. 2015, 71, 1054.
doi: 10.1002/ps.4029 |
| [4] |
Mansfield J.; Genin S.; Magori S.; Citovsky V.; Sriariyanum M.; Ronald P.; Dow M.; Verdier V.; Beer S.V.; Machado M.A.; Toth I.; Salmond G.; Foster G.D. Mol. Plant Pathol. 2012, 13, 614.
doi: 10.1111/mpp.2012.13.issue-6 |
| [5] |
Yang L.; Bao X.P. RSC Adv. 2017, 7, 34005.
doi: 10.1039/C7RA04819J |
| [6] |
Zhang M.; Dai Z.C.; Qian S.S.; Liu J.Y.; Xiao Y.; Lu A.M.; Zhu H.L.; Wang J.X.; Ye Y.H. J. Agric. Food Chem. 2014, 62, 9637.
doi: 10.1021/jf504359p |
| [7] |
Khan I.; Ibrar A.; Abbas N.; Saeed A. Eur. J. Med. Chem. 2014, 76, 193.
doi: 10.1016/j.ejmech.2014.02.005 |
| [8] |
Fan Z.; Shi J.; Bao X. Mol. Diversity 2018, 22, 657.
doi: 10.1007/s11030-018-9821-8 |
| [9] |
Shi J.; Luo N.; Ding M.; Bao X. Chin. Chem. Lett. 2020, 31, 434.
doi: 10.1016/j.cclet.2019.06.037 |
| [10] |
Opoku-Temeng C.; Naclerio G.A.; Mohammad H.; Dayal N.; Abutaleb N.S.; Seleem M.N.; Sintim H.O. Eur. J. Med. Chem. 2018, 155, 797.
doi: S0223-5234(18)30514-2 pmid: 29957525 |
| [11] |
Cui Z.M.; Li Y.S.; Hu D.K.; Tian H.; Jiang J.Z.; Wang Y.; Yan X.J. Sci. Rep. 2016, 6, 20204.
doi: 10.1038/srep20204 |
| [12] |
Wu W.; Fei Q.; He J.; Liu L.; Ouyang G. Chem. Bull. 2019, 82, 74. (in Chinese)
|
|
吴文能, 费强, 何军, 刘雷, 欧阳贵平, 化学通报, 2019, 82, 74.).
|
|
| [13] |
Zhang Y.; Liu X.H.; Zhan Y.Z.; Zhang L.Y.; Li Z.M.; Li Y.H.; Zhang X.; Wang B.L. Bioorg. Med. Chem. Lett. 2016, 26, 4661.
doi: 10.1016/j.bmcl.2016.08.059 |
| [14] |
Wang T.; Miao W.; Wu S.; Bing G.; Zhang X.; Qin Z.; Yu H.; Qin X.; Fang J. Chin. J. Chem. 2011, 29, 959.
doi: 10.1002/cjoc.201190196 |
| [15] |
Chen J.; Chen Y.; Gan X.; Song B.; Hu D.; Song B. J. Agric. Food Chem. 2018, 66, 9616.
doi: 10.1021/acs.jafc.8b03020 |
| [16] |
Han F.; Wan R.; Wang Y.; Wang P.; Wang J. Chin. J. Org. Chem. 2010, 30, 132. (in Chinese)
|
|
韩峰, 万嵘, 王瑶, 王朋, 王锦堂, 有机化学, 2010, 30, 132.).
|
|
| [17] |
Chen X.; Gan X.; Chen J.; Chen Y.; Wang Y.; Hu D.; Song B. Chin. J. Org. Chem. 2017, 37, 2343. (in Chinese)
doi: 10.6023/cjoc201703022 pmid: 4013A66C-29E4-4C33-97B0-76454608F358 |
|
陈学文, 甘秀海, 陈吉祥, 陈永中, 王艳娇, 胡德禹, 宋宝安, 有机化学, 2017, 37, 2343.).
doi: 10.6023/cjoc201703022 pmid: 4013A66C-29E4-4C33-97B0-76454608F358 |
|
| [18] |
Yan L.; Li Y.; Deng M.; Chen A.; Du Z.; Dong C.; Chen H. Chin. J. Org. Chem. 2020, 40, 731. (in Chinese)
doi: 10.6023/cjoc201907052 pmid: 32e327ce-834d-4a95-9fcd-1a877fcad60c |
|
鄢龙家, 黎永良, 邓明高, 陈安超, 杜志云, 董长治, 陈惠雄, 有机化学, 2020, 40, 731.).
doi: 10.6023/cjoc201907052 pmid: 32e327ce-834d-4a95-9fcd-1a877fcad60c |
|
| [19] |
Nazir M.; Abbasi M.A.; Rehman A.; Siddiqui S.Z.; Khan K.M.; Kanwal; Salar, U.; Shahi, M.; Ashraf, M.; Lodhi, M.A.; Khan, F.A.Bioorg. Chem. 2018, 81, 253.
doi: 10.1016/j.bioorg.2018.08.010 |
| [20] |
Xu G.F.; Song B.A.; Bhadury P.S.; Yang S.; Zhang P.Q.; Jin L.H.; Xue W.; Hu D.Y.; Lu P. Bioorg. Med. Chem. 2007, 15, 3768.
doi: 10.1016/j.bmc.2007.03.037 |
| [21] |
Luo H.; Hu D.; Wu J.; He M.; Jin L.; Yang S.; Song B. Int. J. Mol. Sci. 2012, 13, 6730.
doi: 10.3390/ijms13066730 |
| [22] |
Embrechts W.; Herschke F.; Pauwels F.; Stoops B.; Last S.; Pieters S.; Pande V.; Pille G.; Amssoms K.; Smyej I.; Dhuyvetter D.; Scholliers A.; Mostmans W.; Van Dijck K.; Van Schoubroeck B.; Thone T.; De Pooter D.; Fanning G.; Jonckers T. H. M.; Horton H.; Raboisson P.; McGowan D. J. Med. Chem. 2018, 61, 6236.
doi: 10.1021/acs.jmedchem.8b00643 |
| [23] |
Zayed M.F.; Ihmaid S.K.; Ahmed H. E. A.; El-Adl K.; Asiri A.M.; Omar A.M. Molecules 2017, 22, 188.
doi: 10.3390/molecules22020188 |
| [24] |
Vitaku E.; Smith D.T.; Njadarson J.T. J. Med. Chem. 2014, 57, 10257.
doi: 10.1021/jm501100b |
| [25] |
Fan Z.; Shi J.; Luo N.; Ding M.; Bao X. J. Agric. Food Chem. 2019, 67, 11598.
doi: 10.1021/acs.jafc.9b04733 |
| [1] | 冯康博, 陈炯, 古双喜, 王海峰, 陈芬儿. 全连续流反应技术在药物合成中的新进展(2019~2022)[J]. 有机化学, 2024, 44(2): 378-397. |
| [2] | 李鹏辉, 谢青洋, 万福贤, 张元红, 姜林. 含环丙基的新型取代嘧啶-5-甲酰胺的合成及杀菌活性研究[J]. 有机化学, 2024, 44(2): 650-656. |
| [3] | 邹发凯, 王能中, 姚辉, 王慧, 刘明国, 黄年玉. 1β-/3R-芳基硫代糖的区域与立体选择性合成[J]. 有机化学, 2024, 44(2): 593-604. |
| [4] | 李路瑶, 贺忠文, 张振国, 贾振华, 罗德平. 三芳基碳正离子在有机合成中的应用[J]. 有机化学, 2024, 44(2): 421-437. |
| [5] | 梅青刚, 李清寒. 可见光促进C(3)(杂)芳硫基吲哚化合物的合成研究进展[J]. 有机化学, 2024, 44(2): 398-408. |
| [6] | 赵茜帆, 陈永正, 张世明. 碳基非金属催化剂在有机合成领域的应用及机理研究[J]. 有机化学, 2024, 44(1): 137-147. |
| [7] | 陈珊, 陈志林, 胡琼, 蒙艳双, 黄悦, 陶萍芳, 卢丽如, 黄国保. 含双硫脲基团分子钳在非极性溶剂中识别中性分子[J]. 有机化学, 2024, 44(1): 277-281. |
| [8] | 王化坤, 任晓龙, 宣宜宁. 卤盐催化的α,β-环氧羧酸酯与异氰酸酯[3+2]环加成反应研究[J]. 有机化学, 2024, 44(1): 251-258. |
| [9] | 金玉坤, 任保轶, 梁福顺. 可见光介导的三氟甲基的选择性C-F键断裂及其在偕二氟类化合物合成中的应用[J]. 有机化学, 2024, 44(1): 85-110. |
| [10] | 马翠云, 罗海澜, 张福华, 郭丹, 陈树兴, 王飞. 3-Pyrrolyl BODIPY的绿色生物合成、光物理性质及应用研究[J]. 有机化学, 2024, 44(1): 216-223. |
| [11] | 王博珍, 张婕, 粘春惠, 金茗茗, 孔苗苗, 李物兰, 何文斐, 吴建章. 含有3,4-二氯苯基的酰胺类化合物的合成及抗肿瘤活性研究[J]. 有机化学, 2024, 44(1): 232-241. |
| [12] | 杨维清, 葛宴兵, 陈元元, 刘萍, 付海燕, 马梦林. 1,8-萘酰亚胺衍生物的设计、合成及其对半胱氨酸的识别研究[J]. 有机化学, 2024, 44(1): 180-194. |
| [13] | 于士航, 刘嘉威, 安碧玉, 边庆花, 王敏, 钟江春. 黑腹尼虎天牛接触性信息素的不对称合成[J]. 有机化学, 2024, 44(1): 301-308. |
| [14] | 曹瑞霞, 贾玉萍. 含香豆素的吡咯并[2,3-d]嘧啶衍生物的合成及生物活性研究[J]. 有机化学, 2023, 43(9): 3304-3311. |
| [15] | 李焕清, 陈兆华, 陈祖佳, 邱琪雯, 张又才, 陈思鸿, 汪朝阳. 基于有机小分子的汞离子荧光探针研究进展[J]. 有机化学, 2023, 43(9): 3067-3077. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||