化学学报 ›› 2012, Vol. 70 ›› Issue (17): 1791-1797.DOI: 10.6023/A12050262 上一篇 下一篇
研究论文
王康a, 王海龙a, 约翰·马克b, 李文军a, 小林长夫b, 姜建壮a
Wang Kanga, Wang Hailonga, Mack Johnb, Li Wenjuna, Kobayashi Nagaob, Jiang Jianzhuanga
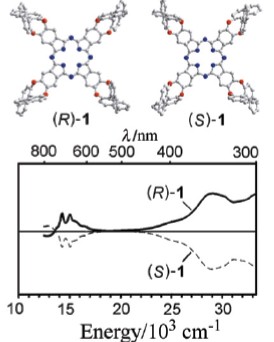
在锂存在的条件下在正戊醇中回流环化四聚相应的光学活性的前驱体联二萘[2,1-e:1',2'-g][1,4]二羟基-5,6-邻苯二氰, 然后用醋酸酸化处理, 合成了一对对映的四个联二萘酚基团取代的手性自由酞菁四(联二萘[1,2-e:1',2'-g]-1,4-二羟基)[2,3-b;2',3'-k;2'',3''-t;2''',3'''-c']酞菁(1), 并利用一系列的光谱学方法以及元素分析表征了这种新型的手性酞菁化合物. 单晶X衍射分析确定了两种对映体的绝对构型, 从而阐明了自由酞菁(1)的手性分配.