化学学报 ›› 2019, Vol. 77 ›› Issue (9): 874-878.DOI: 10.6023/A19050189 上一篇 下一篇
所属专题: 有机自由基化学
研究通讯
投稿日期:2019-05-21
发布日期:2019-06-21
通讯作者:
柳忠全
E-mail:liuzhq@lzu.edu.cn
基金资助:
Xiao, Yingxiaa, Liu, Zhong-Quanab*( )
)
Received:2019-05-21
Published:2019-06-21
Contact:
Liu, Zhong-Quan
E-mail:liuzhq@lzu.edu.cn
Supported by:文章分享
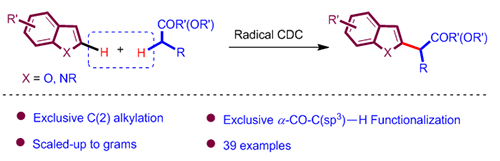
报道了一种小分子酮、酯与富电子杂环芳烃的高度选择性的自由基脱氢交叉偶联反应. 酯、酮作为溶剂, 过氧化物加热条件下发生裂解与酯、酮作用产生α羰基碳中心自由基, 进而与富电子杂环芳烃发生交叉脱氢偶联, 得到一系列C-2官能化富电子杂环产物. 该反应成功地运用自由基的极性效应, 从而精确控制自由基反应的化学选择性. 此外, 该体系还具有反应迅速、操作简便、官能团兼容性较好、区域选择性单一等优点. 预期它将在合成有机化学上得到较广泛的应用.
肖莹霞, 柳忠全. 富电子杂环芳烃与酮酯的自由基脱氢偶联反应[J]. 化学学报, 2019, 77(9): 874-878.
Xiao, Yingxia, Liu, Zhong-Quan. Radical-Promoted Cross Dehydrogenative Coupling of Ketones and Esters with Electron-Rich Heteroarenes[J]. Acta Chimica Sinica, 2019, 77(9): 874-878.
| Entry | Radical initiator | Peroxide (equiv.) | Sol./mL | t/h | Tem./℃ | Yieldb/% |
|---|---|---|---|---|---|---|
| 1 | Fe(acac)3 (10 mol%) | TBHP(decane) (3) | 5 | 12 | 130 | 8 |
| 2 | Fe(acac)3 (10 mol%) | DTBP (3) | 5 | 12 | 130 | 25 |
| 3 | Fe(acac)3 (10 mol%) | K2S2O8 (3) | 5 | 12 | 130 | 0 |
| 4 | Fe(acac)3 (10 mol%) | DCP (3 ) | 5 | 12 | 130 | 39 |
| 5 | Fe(acac)3 (10 mol%) | TBPA (3) | 5 | 1 | 130 | 38 |
| 6 | — | DCP (3 ) | 5 | 12 | 130 | 24 |
| 7 | — | TBPA (3) | 5 | 1 | 130 | 54 |
| 8 | — | TBPA (3) | 4 | 1 | 130 | 45 |
| 9 | — | TBPA (3) | 6 | 1 | 130 | 60 |
| 10 | — | TBPA (3) | 7 | 1 | 130 | 51 |
| 11 | — | TBPA (3) | 6 | 2 | 130 | 47 |
| 12 | — | TBPA (2) | 6 | 1 | 130 | 32 |
| 13 | — | TBPA (3) | 6 | 1 | 120 | 26 |
| Entry | Radical initiator | Peroxide (equiv.) | Sol./mL | t/h | Tem./℃ | Yieldb/% |
|---|---|---|---|---|---|---|
| 1 | Fe(acac)3 (10 mol%) | TBHP(decane) (3) | 5 | 12 | 130 | 8 |
| 2 | Fe(acac)3 (10 mol%) | DTBP (3) | 5 | 12 | 130 | 25 |
| 3 | Fe(acac)3 (10 mol%) | K2S2O8 (3) | 5 | 12 | 130 | 0 |
| 4 | Fe(acac)3 (10 mol%) | DCP (3 ) | 5 | 12 | 130 | 39 |
| 5 | Fe(acac)3 (10 mol%) | TBPA (3) | 5 | 1 | 130 | 38 |
| 6 | — | DCP (3 ) | 5 | 12 | 130 | 24 |
| 7 | — | TBPA (3) | 5 | 1 | 130 | 54 |
| 8 | — | TBPA (3) | 4 | 1 | 130 | 45 |
| 9 | — | TBPA (3) | 6 | 1 | 130 | 60 |
| 10 | — | TBPA (3) | 7 | 1 | 130 | 51 |
| 11 | — | TBPA (3) | 6 | 2 | 130 | 47 |
| 12 | — | TBPA (2) | 6 | 1 | 130 | 32 |
| 13 | — | TBPA (3) | 6 | 1 | 120 | 26 |
| [1] |
For selected recent reviews on CDC reactions, see: (a) Li, C.-J . Acc. Chem. Res. 2009, 42, 335.
doi: 10.1021/ar800164n |
|
(b) Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215.
doi: 10.1021/ar800164n |
|
|
(c) Girard, S. A.; Knauber, T.; Li, C.-J . Angew. Chem., Int. Ed 2014, 53, 74.
doi: 10.1021/ar800164n |
|
|
(d) Jia, F.; Li, Z . Org. Chem. Front 2014, 1, 194.
doi: 10.1021/ar800164n |
|
|
(e) Zhang, J.; Lu, Q.; Liu, C.; Lei, A . Chin. J. Org. Chem 2015, 35, 743.
doi: 10.1021/ar800164n |
|
|
(张剑, 陆庆全, 刘超, 雷爱文, 有机化学, 2015, 35, 743.);
doi: 10.1021/ar800164n |
|
|
(f) Zhang, G.; Bian, C.; Lei, A . Chin. J. Catal 2015, 36, 1428.
doi: 10.1021/ar800164n |
|
|
(g) Pei, P.; Zhang, F.; Yi, H.; Lei, A . Acta Chim. Sinica 2017, 75, 15.
doi: 10.1021/ar800164n |
|
|
(裴朋昆, 张凡, 易红, 雷爱文, 化学学报, 2017, 75, 15.);
doi: 10.1021/ar800164n |
|
|
(h) Shao, A.; Li, N.; Gao, Y.; Zhan, J.; Chiang, C. W.; Lei, A . Chin. J. Chem. 2018, 36, 619;
doi: 10.1021/ar800164n |
|
|
(i) Liu, Y.; Yi, H.; Lei, A . Chin. J. Chem. 2018, 36, 692.
doi: 10.1021/ar800164n |
|
| [2] |
For selected recent reviews on C—H functionalization, see: (a) Zhang, S.; Zhang, F.; Tu, Y.-Q. Chem. Soc. Rev. 2011, 40, 1937.
doi: 10.1039/c0cs00063a |
|
(b) Davies, H. M. L.; Morton, D. Chem. Soc. Rev. 2011, 40, 1857.
doi: 10.1039/c0cs00063a |
|
|
(c) Newhouse, T.; Baran, P. S . Angew. Chem., Int. Ed. 2011, 50, 3362.
doi: 10.1039/c0cs00063a |
|
|
(d) Liu, C.; Zhang, H.; Shi, W.; Lei, A . Chem. Rev. 2011, 111, 1780.
doi: 10.1039/c0cs00063a |
|
|
(e) Engle, K. M.; Mei, T.-S.; Wasa, M.; Yu, J.-Q . Acc. Chem. Res. 2012, 45, 788.
doi: 10.1039/c0cs00063a |
|
|
(f) Roizen, J. L.; Harvey, M. E.Du Bois, J . Acc. Chem. Res. 2012, 45, 911.
doi: 10.1039/c0cs00063a |
|
|
(g) Rouquet, G.; Chatani, N . Angew. Chem., Int. Ed. 2013, 52, 11726.
doi: 10.1039/c0cs00063a |
|
|
(h) He, J.; Wasa, M.; Chan, K. S. L.; Shao, Q.; Yu, J.-Q . Chem. Rev. 2017, 117, 8754.
doi: 10.1039/c0cs00063a |
|
|
(i) Le Bras, J.; Muzart, J . Chem. Rev. 2011, 111, 1170.
doi: 10.1039/c0cs00063a |
|
|
(j) Sun, C.-L.; Li, B.-J.; Shi, Z.-J . Chem. Rev. 2011, 111, 1293.
doi: 10.1039/c0cs00063a |
|
|
(k) Cho, S. H.; Kim, J. Y.; Kwak, J.; Chang, S . Chem. Soc. Rev. 2011, 40, 5068.
doi: 10.1039/c0cs00063a |
|
|
(l) Shang, X.; Liu, Z.-Q . Chem. Soc. Rev. 2013, 42, 3253;
doi: 10.1039/c0cs00063a |
|
|
(m) Yang, L.; Huang, H . Chem. Rev. 2015, 115, 3468.
doi: 10.1039/c0cs00063a |
|
|
(n) Guo, X.-X.; Gu, D.-W.; Wu, Z.; Zhang, W . Chem. Rev. 2015, 115, 1622.
doi: 10.1039/c0cs00063a |
|
|
(o) Zheng, Q.-Z.; Jiao, N . Chem. Soc. Rev. 2016, 45, 4590.
doi: 10.1039/c0cs00063a |
|
|
(p) Murakami, K.; Yamada, S.; Kaneda, T.; Itami, K . Chem. Rev. 2017, 117, 9302.
doi: 10.1039/c0cs00063a |
|
|
(q) Yuan, S.; Wang, Y.; Qiu, G.; Liu, J . Chin. J. Org. Chem. 2017, 37, 566.
doi: 10.1039/c0cs00063a |
|
|
(袁斯甜, 王艳华, 邱观音生, 刘晋彪, 有机化学, 2017, 37, 566.);
doi: 10.1039/c0cs00063a |
|
|
(r) Zhang, J. J.; Cheng, Y. B.; Duan, X. H . Chin. J. Chem. 2017, 35, 311.
doi: 10.1039/c0cs00063a |
|
|
(s) Ruan, L.; Chen, C.; Zhang, X.; Sun, J . Chin. J. Org. Chem. 2018, 38, 3155.
doi: 10.1039/c0cs00063a |
|
|
(阮利衡, 陈春欣, 张晓欣, 孙京, 有机化学, 2018, 38, 3155.);
doi: 10.1039/c0cs00063a |
|
|
(t) Zhang, X.; Li, P.; Yuan, Y.; Jia, X . Chin. J. Org. Chem. 2018, 38, 2435.
doi: 10.1039/c0cs00063a |
|
|
(张学文, 李鹏飞, 袁宇, 贾晓东, 有机化学, 2018, 38, 2435.);
doi: 10.1039/c0cs00063a |
|
|
(u) Gu, Z.; Ji, S . Acta. Chim. Sinica 2018, 76, 347.
doi: 10.1039/c0cs00063a |
|
|
(顾正祥, 纪顺俊, 化学学报, 2018, 76, 347.)
doi: 10.1039/c0cs00063a |
|
| [3] |
For selected recent reviews, see: (a) Shang, X.; Liu, Z.-Q . Acta Chim. Sinica 2015, 73, 1275.
doi: 10.6023/A15060407 |
|
( 尚筱洁, 柳忠全 , 化学学报, 2015, 73, 1275.)
doi: 10.6023/A15060407 |
|
|
(b) Yi, H.; Zhang, G.; Wang, H.; Huang, Z.; Wang, J.; Singh, A. K.; Lei, A. Chem. Rev. 2017, 117, 9016.
doi: 10.6023/A15060407 |
|
| [4] |
(a) Harris, E. F. P.; Waters, W. A . Nature 1952, 170, 212.
doi: 10.1038/170212a0 |
|
(b) Walling, C. Pure Appl. Chem. 1967, 15, 69.
doi: 10.1038/170212a0 |
|
|
(c) Tedder, J. M. Angew. Chem. Int. Ed. Engl. 1982, 21, 401.
doi: 10.1038/170212a0 |
|
|
(d) Giese, B . Angew. Chem. Int. Ed. Engl. 1989, 28, 969.
doi: 10.1038/170212a0 |
|
|
(e) Roberts, B. P . Chem. Soc. Rev. 1999, 28, 25.
doi: 10.1038/170212a0 |
|
| [5] |
Ravelli, D.; Fagnoni, M.; Fukuyama, T.; Nishikawa, T.; Ryu, I . ACS Catal. 2018, 8, 701.
doi: 10.1021/acscatal.7b03354 |
| [6] |
Tian, Y.; Sun, C.; Tan, R. X.; Liu, Z.-Q . Green Chem. 2018, 20, 588.
doi: 10.1039/C7GC03745G |
| [7] |
(a) Snider, B. B. Chem. Rev. 1996, 96, 339.
doi: 10.1021/cr950026m |
|
(b) Heiba E. I.; Dessau, R. M. J. Am. Chem. Soc. 1971, 93, 524.
doi: 10.1021/cr950026m |
|
|
(c) Iwahama, T.; Sakaguchi, S. Ishii, Y. Chem. Commun. 2000, 2317.
doi: 10.1021/cr950026m |
|
|
(d) Linker, U.; Kersten, B.; Linker, T . Tetrahedron 1995, 51, 9917.
doi: 10.1021/cr950026m |
|
|
(e) Xie, J.; Huang, Z.-Z . Chem. Commun. 2010, 46, 1947.
doi: 10.1021/cr950026m |
|
|
(f) Zhu, L.; Chen, H.; Wang, Z.; Li, C . Org. Chem. Front. 2014, 1, 1299.
doi: 10.1021/cr950026m |
|
|
(g) Schweitzer-Chaput, B.; Demaerel, J.; Engler, H.; Klussmann, M . Angew. Chem., Int. Ed. 2014, 53, 8737.
doi: 10.1021/cr950026m |
|
|
(h) Chu, X.; Meng, H.; Zi, Y.; Xu, X.-P.; Ji, S.-J . Chem. -Eur. J. 2014, 20, 17198.
doi: 10.1021/cr950026m |
|
|
(i) Lan, X.; Wang, N.-X.; Zhang, W.; Wen, J.; Bai, C.; Xing, Y.-L.; Li, Y.-H . Org. Lett. 2015, 17, 4460.
doi: 10.1021/cr950026m |
|
|
(j) Shiraishi, Y.; Tsukamoto, D.; Hirai, T . Org. Lett. 2008, 10, 3117.
doi: 10.1021/cr950026m |
|
|
(k) Tsukamoto, D.; Shiraishi, Y.; Hirai, T . J. Org. Chem. 2010, 75, 1450.
doi: 10.1021/cr950026m |
|
| [8] | (a) Liu, Z.-Q.; Li, Z. Chem . Commun. 2016, 52, 14278. |
| (b) Xu, Z.; Hang, Z.; Chai, L.; Liu, Z.-Q. Org . Lett. 2016, 18, 4662. |
| [1] | 易敬霖, 陈茂. 三氟氯乙烯与甲基异丙烯基醚的光诱导共聚反应★[J]. 化学学报, 2024, 82(2): 126-131. |
| [2] | 李雅宁, 王晓艳, 唐勇. 自由基聚合的立体选择性调控★[J]. 化学学报, 2024, 82(2): 213-225. |
| [3] | 李珊, 路俊欣, 刘杰, 蒋绿齐, 易文斌. 氟烷基亚磺酸钠盐电化学合成α-氟烷基酮[J]. 化学学报, 2024, 82(2): 110-114. |
| [4] | 邓沈娜, 彭常春, 牛云宏, 许云, 张云霄, 陈祥, 王红敏, 刘珊珊, 沈晓. 自由基Brook重排调控的α-氟烷基-α-硅基甲醇参与的烯烃双官能团化反应[J]. 化学学报, 2024, 82(2): 119-125. |
| [5] | 陈健强, 朱钢国, 吴劼. 镍催化氮杂环丙烷的开环偶联反应研究[J]. 化学学报, 2024, 82(2): 190-212. |
| [6] | 任妍妍, 李欣, 韩英锋. 基于氮杂环卡宾蓝光有机自由基的合成及其光学性质研究★[J]. 化学学报, 2023, 81(7): 735-740. |
| [7] | 刘坜, 郑刚, 范国强, 杜洪光, 谭嘉靖. 4-酰基/氨基羰基/烷氧羰基取代汉斯酯参与的有机反应研究进展[J]. 化学学报, 2023, 81(6): 657-668. |
| [8] | 杨洁, 凌琳, 李玉学, 吕龙. 高氯酸铵热分解机理的密度泛函理论研究[J]. 化学学报, 2023, 81(4): 328-337. |
| [9] | 赵亚婷, 刘帆, 汪秋安, 夏吾炯. 可见光促进(氮杂)芳香胺与重氮乙酸乙酯的N-烷基化反应[J]. 化学学报, 2023, 81(2): 111-115. |
| [10] | 陈健强, 朱钢国, 吴劼. 草酸酯类化合物在自由基脱羟基化反应中的研究进展[J]. 化学学报, 2023, 81(11): 1609-1623. |
| [11] | 张红丹, 兰欣雨, 程鹏. 羟基自由基辅助沸石分子筛合成的研究进展[J]. 化学学报, 2023, 81(1): 100-110. |
| [12] | 李小娟, 叶梓瑜, 谢书涵, 王永净, 王永好, 吕源财, 林春香. 氮氯共掺杂多孔碳活化过一硫酸盐降解苯酚的性能及机理研究[J]. 化学学报, 2022, 80(9): 1238-1249. |
| [13] | 岳广禄, 魏婧瑶, 邱頔, 莫凡洋. 芳基锡烷的合成研究进展[J]. 化学学报, 2022, 80(7): 956-969. |
| [14] | 吴波, 王冲, 李宝林, 王春儒. 光驱动的金属富勒烯分子磁开关※[J]. 化学学报, 2022, 80(2): 101-104. |
| [15] | 杨民, 叶柏柏, 陈健强, 吴劼. 可见光催化烷基磺酰自由基启动芳酰肼的烷基磺酰化反应[J]. 化学学报, 2022, 80(1): 11-15. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||