化学学报 ›› 2024, Vol. 82 ›› Issue (1): 36-45.DOI: 10.6023/A23090412 上一篇 下一篇
研究论文
李萍a,b, 杨琪玉a, 曾婧a, 张然a, 陈秋燕a, 闫飞a,b,*( )
)
投稿日期:2023-09-13
发布日期:2023-11-29
基金资助:
Ping Lia,b, Qiyu Yanga, Jing Zenga, Ran Zhanga, Qiuyan Chena, Fei Yana,b( )
)
Received:2023-09-13
Published:2023-11-29
Contact:
E-mail: Supported by:文章分享

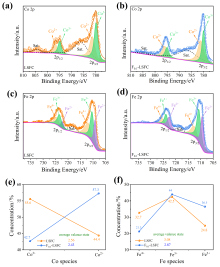
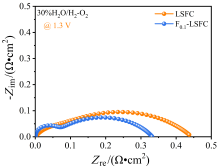
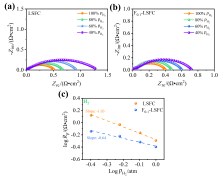
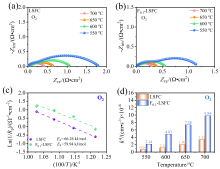
可逆固体氧化物电池(RSOC)表现出优异的热力学和动力学性质, 被认为是一种很有前途的能量转换装置. 制备了两种RSOC电极材料La0.6Sr0.4Fe0.8Co0.2O3 (LSFC)和La0.6Sr0.4Fe0.8Co0.2F0.1O2.9 (F0.1-LSFC), 对比了F掺杂对电池放电和电解性能的影响并对电极表面动力学反应进行探究. 研究表明F掺杂可降低B位元素价态、提高材料氧空位浓度, 进而提高电池性能. 700 ℃, 30%H2O/H2燃料下, 由F0.1-LSFC组成的RSOC的最大功率密度为234.3 mW•cm-2, 约为LSFC组成的RSOC的1.7倍. 并且在1.3 V下, 由LSFC和F0.1-LSFC组成的RSOC的电流密度分别为–245.6和–417.9 mA•cm-2. 此外, 通过电极表面动力学分析发现, 对于氢氧化反应(HOR), F0.1-LSFC电极反应的速度控制步骤(RDS)主要是电荷转移反应, 而LSFC电极反应的RDS主要是氢气的吸附和解离反应; 对于氧还原反应(ORR), RDS是吸附的氧原子还原成O-.
李萍, 杨琪玉, 曾婧, 张然, 陈秋燕, 闫飞. 氟掺杂对可逆固体氧化物电池性能的影响及相关动力学研究[J]. 化学学报, 2024, 82(1): 36-45.
Ping Li, Qiyu Yang, Jing Zeng, Ran Zhang, Qiuyan Chen, Fei Yan. Effect of Fluorine Doping on the Performance of Reversible Solid Oxide Cells and Related Kinetic Studies[J]. Acta Chimica Sinica, 2024, 82(1): 36-45.











| [1] |
Rehman, S. U.; Hassan, M. H.; Kim, H. S.; Song, R. H.; Lim, T. H.; Hong, J. E.; Joh, D. W.; Park, S. J.; Lee, J. W.; Lee, S. B. Appl. Catal. B 2023, 333, 122784.
doi: 10.1016/j.apcatb.2023.122784 |
| [2] |
Yang, X.-X.; Miao, H.; Yuan, J.-L. Chem. Ind. Eng. Prog. 2021, 40, 4904. (in Chinese)
|
|
(杨晓幸, 苗鹤, 袁金良, 化工进展, 2021, 40, 4904.)
doi: 10.16085/j.issn.1000-6613.2021-0594 |
|
| [3] |
Pei, K.; Luo, S.-R.; He, F.; Arbiol, J.; Xu, Y.-S.; Zhu, F.; Wang, Y.-K.; Chen, Y. Appl. Catal. B 2023, 330, 122601.
doi: 10.1016/j.apcatb.2023.122601 |
| [4] |
Wei, K.-Y.; Zhao, Y.; Chen, K.-Y.; Sun, K.; Wu, T.; Dong, Z.-H.; Li, C.-L. Adv. Funct. Mater. 2021, 31, 2009133.
doi: 10.1002/adfm.v31.18 |
| [5] |
Tian, Y.-F.; Wang, W.-J.; Liu, Y.; Naden, A.; Xu, M.; Wu, S.-T.; Chi, B.; Pu, J.; Irvine, J. T. S. ACS Catal. 2021, 11, 3704.
doi: 10.1021/acscatal.0c05543 |
| [6] |
Tian, Y.-F.; Yang, S.-C.; Li, Y.-T.; Zhang, M.-Y.; Gu, S.-D.; Zheng, K.-Q.; Wang, X.-X.; Ling, Y.-H.; Pu, J.; Chi, B. J. Fuel Chem. Technol. 2022, 50, 1638. (in Chinese)
doi: 10.1016/S1872-5813(22)60049-5 |
|
(田云峰, 杨偲晨, 李宜桐, 章梦云, 顾水丹, 郑克晴, 王鑫鑫, 凌意瀚, 蒲健, 池波, 燃料化学学报, 2022, 50, 1638.)
|
|
| [7] |
Wang, Z.-H.; Meng, Y.-J.; Singh, M.; Jing, Y.-F.; Asghar, M. I.; Lund, P.; Fan, L.-D. ACS Appl. Mater. Interfaces 2023, 15, 870.
doi: 10.1021/acsami.2c16002 |
| [8] |
Yang, Z.-B.; Zhang, P.-P.; Lei, Z.; Ge, B.; Peng, S.-P. J. Chin. Ceram. Soc. 2021, 49, 56. (in Chinese)
|
|
(杨志宾, 张盼盼, 雷泽, 葛奔, 彭苏萍, 硅酸盐学报, 2021, 49, 56.)
|
|
| [9] |
Chen, K.; Zhang, Y.; Li, C.-L. ACS Nano. 2018, 12, 12444.
doi: 10.1021/acsnano.8b06660 |
| [10] |
Shao, K.; Li, F.-J.; Zhang, G.-H.; Zhang, Q.-L.; Maliutina, K.; Fan, L.-D. ACS Appl. Mater. Interfaces 2019, 11, 27924.
doi: 10.1021/acsami.9b08448 |
| [11] |
Li, P.; Liu, F.; Wei, W.; Yang, B.-B.; Ma, X.-Y.; Yan, F.; Gan, T.; Fu, D. Ind. Eng. Chem. Res. 2022, 61, 13795.
doi: 10.1021/acs.iecr.2c02282 |
| [12] |
Xu, N.; Sun, M.-Z.; Geng, D.; Yu, L.-J.; Xu, Z.-L. Rare Met. Mater. Eng. 2021, 50, 3885. (in Chinese)
|
|
(徐娜, 孙梦真, 耿多, 于龙娇, 徐占林, 稀有金属材料与工程, 2021, 50, 3885.)
|
|
| [13] |
Li, P.; Liu, F.; Yang, B.-B.; Wei, W.; Ma, X.-Y.; Yan, F.; Gan, T.; Fu, D. Electrochim. Acta 2023, 446, 142069.
doi: 10.1016/j.electacta.2023.142069 |
| [14] |
Liu, Z.; Tang, Z.-J.; Song, Y.-F.; Yang, G.-M.; Qian, W.-R.; Yang, M.-T.; Shao, Z.-P. Nano-Micro Lett. 2022, 14, 217.
doi: 10.1007/s40820-022-00967-6 |
| [15] |
Cao, D.-P.; Yao, Z.-G.; Liu, J.-J.; Zhang, J.-C.; Li, C.-L. Energy Storage Mater. 2018, 11, 152.
|
| [16] |
Wang, S.; Jiang, H.-G.; Gu, Y.-C.; Yin, B.; Chen, S.-H.; Shen, M.-Y.; Zheng, Y.-F.; Ge, L.; Chen, H.; Guo, L.-C. Electrochim. Acta 2020, 337, 135794.
doi: 10.1016/j.electacta.2020.135794 |
| [17] |
Liu, C.-L.; Ma, B.; Zhou, Y.-K.; Wu, K.-M. J. Ceram. 2022, 43, 870. (in Chinese)
|
|
(刘楚林, 马奔, 周盈科, 吴开明, 陶瓷学报, 2022, 43, 870.)
|
|
| [18] |
Fan, S.-S.; Lei, M.; Wu, H.; Hu, J.-L.; Yin, C.-L.; Liang, T.-X.; Li, C.-L. Energy Storage Mater. 2020, 31, 87.
|
| [19] |
Hou, N.-J.; Gan, J.-J.; Yan, Q.-S.; Zhao, Y.-C.; Li, Y.-D. J. Power Sources 2022, 521, 230932.
doi: 10.1016/j.jpowsour.2021.230932 |
| [20] |
Zhang, S.; Yang, C.; Jiang, Y.; Li, P.; Xia, C.-R. J. Power Sources 2023, 77, 300.
|
| [21] |
Li, Y.-H.; Li, Y.; Wan, Y.-H.; Xie, Y.; Zhu, J.-F.; Pan, H.-B.; Zheng, X.-S.; Xia, C.-R. Adv. Energy Mater. 2019, 9, 1803156.
doi: 10.1002/aenm.v9.3 |
| [22] |
Zhang, S.; Jiang, Y.; Han, H.; Li, Y.; Xia, C. ACS Appl. Mater. Interfaces 2022, 15, 28854.
|
| [23] |
Wu, Y.-J.; Yang, Y.-Y.; Zhou, S.-J.; Zhu, W.-W.; Song, W.-C.; Bao, H.; Chen, H.; Ou, X.-M.; Khan, M.; Ling, Y.-H. Ceram. Int. 2020, 46, 6714.
doi: 10.1016/j.ceramint.2019.11.160 |
| [24] |
Ye, L.; Shang, Z.; Xie, K. Angew. Chem. Int. Ed. 2022, 61, e202207211.
doi: 10.1002/anie.v61.32 |
| [25] |
Donazzi, A.; Pelosato, R.; Cordaro, G.; Stucchi, D.; Cristiani, C.; Dotelli, G.; Sora, I. N. Electrochim. Acta 2015, 182, 573.
doi: 10.1016/j.electacta.2015.09.117 |
| [26] |
Park, S.; Han, H.; Yoon, W.; Choi, J.; Kim, Y.; Kim, H.; Kim, W. B. ACS Sustainable Chem. Eng. 2020, 8, 6564.
doi: 10.1021/acssuschemeng.0c01774 |
| [27] |
Dong, X.; Tian, L.; Li, J.; Zhao, Y.-C.; Tian, Y.; Li, Y.-D. J. Power Sources 2014, 249, 270.
doi: 10.1016/j.jpowsour.2013.10.045 |
| [28] |
Yang, X.-X.; Sun, W.; Ma, M.-J.; Xu, C.-M.; Ren, R.-Z.; Qiao, J.-S.; Wang, Z.-H.; Zhen, S.-Y.; Sun, K.-N. J. Power Sources 2021, 60, 7826.
|
| [29] |
Liu, Y.; Tian, Y.-F.; Wang, W.-j.; Li, Y.-T.; Chattopadhyay, S.; Chi, B.; Pu, J. ACS Appl. Mater. Interfaces 2020, 12, 57941.
doi: 10.1021/acsami.0c18583 |
| [30] |
Zhi, X.-J.; Gan, T.; Hou, N.-J.; Fan, L.-J.; Yao, T.-T.; Wang, J.; Zhao, Y.-C.; Li, Y.-D. J. Power Sources 2019, 423, 290.
doi: 10.1016/j.jpowsour.2019.03.088 |
| [31] |
Zhao, Z.; Wang, X.-L.; Tang, S.; Cheng, M.-J.; Shao, Z.-G. Int. J. Hydrogen Energy 2021, 46, 25332.
doi: 10.1016/j.ijhydene.2021.05.072 |
| [32] |
Wang, N.; Tang, C.-M.; Du, L.; Zhu, R.-J.; Xing, L.-X.; Song, Z.-Q.; Yuan, B.-Y.; Zhao, L.; Aoki, Y.; Ye, S. Adv. Energy Mater. 2022, 12, 2201882.
doi: 10.1002/aenm.v12.34 |
| [33] |
Wang, Z.; Yang, W.-Q.; Shafi, S. P.; Bi, L.; Wang, Z.-B.; Peng, R.-R.; Xia, C.-R.; Liu, W.; Lu, Y.-L. J. Mater. Chem. A 2015, 3, 8405.
doi: 10.1039/C5TA00391A |
| [34] |
Song, Y.-F.; Chen, Y.-B.; Wang, W.; Zhou, C.; Zhong, Y.; Yang, G.; Zhou, W.; Liu, M.-L.; Shao, Z.-P. Joule. 2019, 3, 2842.
doi: 10.1016/j.joule.2019.07.004 |
| [35] |
Wang, Z.-Q.; Yang, W.-Q.; Shafi, S. P.; Bi, L.; Wang, Z.-B.; Peng, R.-R.; Lu, Y.-L. J. Mater. Chem. A 2015, 3, 8405.
doi: 10.1039/C5TA00391A |
| [36] |
Li, P.; Yu, B.-L.; Li, J.; Yao, X.-L.; Zhao, Y.-C.; Li, Y.-D. J. Power Sources 2016, 320, 251.
doi: 10.1016/j.jpowsour.2016.04.100 |
| [37] |
Zheng, Y.; Zhao, C.-C.; Li, Y.-F.; Zhang, W.-Q.; Wu, T.; Wang, Z.-C.; Li, Z.-P.; Chen, J.; Wang, J.-C.; Yu, B.; Zhang, J.-J. Nano Energy 2020, 78, 105236.
doi: 10.1016/j.nanoen.2020.105236 |
| [1] | 刘士琨, 邓程维, 姬峰, 闵宇霖, 李和兴. 高温质子交换膜燃料电池中阴极双催化层孔结构的设计研究★[J]. 化学学报, 2023, 81(9): 1135-1141. |
| [2] | 闫绍兵, 焦龙, 何传新, 江海龙. ZIF-67/石墨烯复合物衍生的氮掺杂碳限域Co纳米颗粒用于高效电催化氧还原[J]. 化学学报, 2022, 80(8): 1084-1090. |
| [3] | 王丹, 封波, 张晓昕, 刘亚楠, 裴燕, 乔明华, 宗保宁. 基于热解ZIF-8的氮掺杂碳电化学氧还原合成过氧化氢催化剂[J]. 化学学报, 2022, 80(6): 772-780. |
| [4] | 耿元昊, 林小秋, 孙亚昕, 李惠雨, 秦悦, 李从举. 双金属导电金属有机框架材料Ni/Co-CAT的制备及其氧还原催化性能研究[J]. 化学学报, 2022, 80(6): 748-755. |
| [5] | 李燕丽, 于丹丹, 林森, 孙东飞, 雷自强. α-MnO2纳米棒/多孔碳正极材料的制备及水系锌离子电池性能研究[J]. 化学学报, 2021, 79(2): 200-207. |
| [6] | 梁其梅, 郭昱娇, 郭俊明, 向明武, 刘晓芳, 白玮, 宁平. 亚微米去顶角八面体LiNi0.08Mn1.92O4正极材料制备及高温电化学性能[J]. 化学学报, 2021, 79(12): 1526-1533. |
| [7] | 鲁效庆, 曹守福, 魏晓飞, 李邵仁, 魏淑贤. S掺杂Fe-NC单原子催化剂氧还原机理研究[J]. 化学学报, 2020, 78(9): 1001-1006. |
| [8] | 税子怡, 何娜娜, 陈黎, 赵炜, 陈曦. 多孔钙钛矿型氧还原催化剂在柔性铝空气电池中的应用研究[J]. 化学学报, 2020, 78(6): 557-564. |
| [9] | 渠璐平, 任彤, 王宁, 史月丽, 庄全超. 硬碳材料电极首周嵌钠过程的电化学阻抗谱研究[J]. 化学学报, 2019, 77(7): 634-640. |
| [10] | 宋学霞, 李继成, 李朝晖, 李喜飞, 丁燕怀, 肖启振, 雷钢铁. 钾掺杂对钒酸钠纳米片储钠性能的影响[J]. 化学学报, 2019, 77(7): 625-633. |
| [11] | 常世磊, 梁风, 姚耀春, 马文会, 杨斌, 戴永年. 金属二氧化碳电池的研究进展[J]. 化学学报, 2018, 76(7): 515-525. |
| [12] | 郑媛, 罗静, 魏玮, 刘晓亚. Pickering乳液法制备聚苯胺-石墨烯空心微球[J]. 化学学报, 2017, 75(4): 391-397. |
| [13] | 刘清朝, 马诗喻, 徐吉静, 李中军, 张新波. 锂-空气二次电池关键材料与器件的设计与制备[J]. 化学学报, 2017, 75(2): 137-146. |
| [14] | 杨春, 龚正良, 赵文高, 杨勇. 富锂正极材料xLi3NbO4·(1-x)LiMO2(M=Mn,Co;0 < x < 1)的制备及电化学性能研究[J]. 化学学报, 2017, 75(2): 212-217. |
| [15] | 陈鑫, 鄢慧君, 夏定国. 锗纳米管催化的氧还原反应:催化性能和机理研究[J]. 化学学报, 2017, 75(2): 189-192. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||