化学学报 ›› 2011, Vol. 69 ›› Issue (18): 2117-2122. 上一篇 下一篇
研究论文
邹少爽,陶占良*,陈军
ZOU Shao-Shuang, TAO Zhan-Liang, CHEN Jun
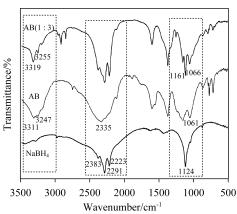
以NaBH4为硼源、氨基络合物Ni(NH3)6Cl2为氨源制备高储氢容量的氨硼烷(NH3BH3, Ammonia Borane, AB)及其放氢性能研究. 通过XRD, FTIR, 11B NMR, ICP等手段分析表征了所制备产物的组成和纯度, 在此基础上探究了原料比例、反应温度、时间和溶剂等因素对产物的影响. 同时, 对不同原料比制得氨硼烷的热解放氢性能进行了研究. 实验结果表明: 当物质的量NaBH4∶Ni(NH3)6Cl2=2∶1经过10 h的反应, 得到了纯度非常高的氨硼烷(纯度>99%)|以NaBH4∶Ni(NH3)6Cl2=3∶1得到的氨硼烷, 当以2 ℃/min进行升温时, 氢气释放主要集中在第一步, 并且没有硼烷和硼嗪等杂质气体的产生. 另外, 在产物中得到了金属Ni纳米颗粒, 经洗涤干燥后其粒径大小可控制在10 nm左右, 在催化氨硼烷等材料的水解放氢方面具有潜在的应用价值.