化学学报 ›› 2021, Vol. 79 ›› Issue (6): 742-746.DOI: 10.6023/A21050198 上一篇 下一篇
研究通讯
杨普苏a, 刘晨旭a, 张文文a,b, 游书力a,b,*( )
)
投稿日期:2021-05-08
发布日期:2021-06-11
通讯作者:
游书力
基金资助:
Pusu Yanga, Chen-Xu Liua, Wen-Wen Zhanga,b, Shu-Li Youa,b( )
)
Received:2021-05-08
Published:2021-06-11
Contact:
Shu-Li You
Supported by:文章分享
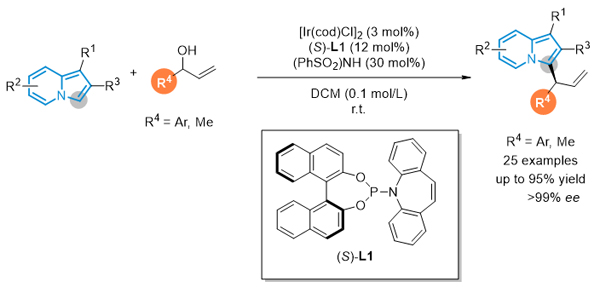
本工作报道了金属铱催化烯丙醇与中氮茚衍生物Friedel-Crafts类型的不对称烯丙基取代反应. 该方法在温和条件下, 以优秀的收率以及对映选择性控制合成了一系列C3位烯丙基化的中氮茚衍生物, 为构建手性中氮茚化合物提供了一条新策略.
杨普苏, 刘晨旭, 张文文, 游书力. 铱催化中氮茚衍生物的Friedel-Crafts类型不对称烯丙基取代反应[J]. 化学学报, 2021, 79(6): 742-746.
Pusu Yang, Chen-Xu Liu, Wen-Wen Zhang, Shu-Li You. Ir-Catalyzed Enantioselective Friedel-Crafts Type Allylic Substitution of Indolizines[J]. Acta Chimica Sinica, 2021, 79(6): 742-746.
| Entry | Additive | Ligand | x mol% | Solvent | NMR yieldb/% | ee c/% |
|---|---|---|---|---|---|---|
| 1 | None | L1 | 0 | DCM | trace | N.D.d |
| 2 | Zn(OTf)2 | L1 | 100 | DCM | >95 | 98 |
| 3 | Fe(OTf)2 | L1 | 100 | DCM | >95 | 99 |
| 4 | Y(OTf)3 | L1 | 100 | DCM | >95 | 99 |
| 5 | 3,5-Cl2C6H3CO2H | L1 | 100 | DCM | 94 | >99 |
| 6 | (PhO)2PO2H | L1 | 100 | DCM | 95 | >99 |
| 7 | (PhSO2)2NH | L1 | 100 | DCM | >95 | >99 |
| 8 | (PhSO2)2NH | L1 | 100 | THF | >95 | 98 |
| 9 | (PhSO2)2NH | L1 | 100 | MeCN | >95 | 95 |
| 10 | (PhSO2)2NH | L1 | 100 | toluene | >95 | 98 |
| 11 | (PhSO2)2NH | L1 | 30 | DCM | >95 | >99 |
| 12 | (PhSO2)2NH | L1 | 10 | DCM | 74 | >99 |
| 13 | (PhSO2)2NH | L2 | 30 | DCM | 68 | >99 |
| 14 | (PhSO2)2NH | L3 | 30 | DCM | >95 | >99 |
| 15e | (PhSO2)2NH | L1 | 30 | DCM | 94 | >99 |
| Entry | Additive | Ligand | x mol% | Solvent | NMR yieldb/% | ee c/% |
|---|---|---|---|---|---|---|
| 1 | None | L1 | 0 | DCM | trace | N.D.d |
| 2 | Zn(OTf)2 | L1 | 100 | DCM | >95 | 98 |
| 3 | Fe(OTf)2 | L1 | 100 | DCM | >95 | 99 |
| 4 | Y(OTf)3 | L1 | 100 | DCM | >95 | 99 |
| 5 | 3,5-Cl2C6H3CO2H | L1 | 100 | DCM | 94 | >99 |
| 6 | (PhO)2PO2H | L1 | 100 | DCM | 95 | >99 |
| 7 | (PhSO2)2NH | L1 | 100 | DCM | >95 | >99 |
| 8 | (PhSO2)2NH | L1 | 100 | THF | >95 | 98 |
| 9 | (PhSO2)2NH | L1 | 100 | MeCN | >95 | 95 |
| 10 | (PhSO2)2NH | L1 | 100 | toluene | >95 | 98 |
| 11 | (PhSO2)2NH | L1 | 30 | DCM | >95 | >99 |
| 12 | (PhSO2)2NH | L1 | 10 | DCM | 74 | >99 |
| 13 | (PhSO2)2NH | L2 | 30 | DCM | 68 | >99 |
| 14 | (PhSO2)2NH | L3 | 30 | DCM | >95 | >99 |
| 15e | (PhSO2)2NH | L1 | 30 | DCM | 94 | >99 |
| [1] |
Sharma, V.; Kumar, V. Med. Chem. Res. 2014, 23, 3593.
doi: 10.1007/s00044-014-0940-1 |
| [2] |
Wall, M. E.; Wani, M. C.; Cook, C. E.; Palmer, K. H.; Mcphail, A. T.; Sim, G. A. J. Am. Chem. Soc. 1966, 16, 3888.
|
| [3] |
(a) Gundersen, L.-L.; Negussie, A. H.; Rise, F.; Østby, O. B. Arch. Pharm. 2003, 336, 191.
doi: 10.1002/ardp.200390019 pmid: 26994846 |
|
(b) Huang, W.; Zuo, T.; Jin, H.; Liu, Z.; Yang, Z.; Yu, X.; Zhang, L.; Zhang, L. Mol. Diversity 2013, 17, 221.
doi: 10.1007/s11030-013-9424-3 pmid: 26994846 |
|
|
(c) Xue, Y.; Tang, J.; Ma, X.; Li, Q.; Xie, B.; Hao, Y.; Jin, H.; Wang, K.; Zhang, G.; Zhang, L.; Zhang, L. Eur. J. Med. Chem. 2016, 115, 94.
doi: 10.1016/j.ejmech.2016.03.016 pmid: 26994846 |
|
|
(d) Park, S.; Kim, E. H.; Kim, J.; Kim, S. H.; Kim, I. Eur. J. Med. Chem. 2018, 144, 435.
doi: 10.1016/j.ejmech.2017.12.056 pmid: 26994846 |
|
| [4] |
(a) Katritzky, A. R.; Qiu, G.; Yang, B.; He, H.-Y. J. Org. Chem. 1999, 64, 7618.
doi: 10.1021/jo9906936 |
|
(b) Zhang, L.; Liang, F.; Sun, L.; Hu, Y.; Hu, H. Synthesis 2000, 12, 1733.
|
|
|
(c) Bora, U.; Saikia, A.; Boruah, R. C. Org. Lett. 2003, 5, 435.
doi: 10.1021/ol020238n |
|
|
(d) Fang, X.; Wu, Y.-M.; Deng, J.; Wang, S.-W. Tetrahedron 2004, 60, 5487.
doi: 10.1016/j.tet.2004.04.012 |
|
|
(e) Rotaru, A. V.; Druta, I. D.; Oeser, T.; Muller, T. J. J. Helv. Chim. Acta 2005, 88, 1798.
doi: 10.1002/(ISSN)1522-2675 |
|
|
(f) Smith, C. R.; Bunnelle, E. M.; Rhodes, A. J.; Sarpong, R. Org. Lett. 2007, 9, 1169.
doi: 10.1021/ol0701971 |
|
|
(g) Xia, J.-B.; You, S.-L. Org. Lett. 2009, 11, 1187.
doi: 10.1021/ol9000872 |
|
|
(h) Muthusaravanan, S.; Perumal, S.; Yogeeswari, P.; Sriram, D. Tetrahedron Lett. 2010, 51, 6439.
doi: 10.1016/j.tetlet.2010.09.128 |
|
|
(i) Zhu, H.; Stockigt, J.; Yu, Y.; Zou, H. Org. Lett. 2011, 13, 2792.
doi: 10.1021/ol200883w |
|
|
(j) Jung, Y.; Kim, I. Tetrahedron 2012, 68, 8198.
doi: 10.1016/j.tet.2012.07.068 |
|
|
(k) Lee, J. H.; Kim, I. J. Org. Chem. 2013, 78, 1283.
doi: 10.1021/jo302590a |
|
|
(l) Liang, Y.; Teng, L.; Wang, Y.; He, Q.; Cao, H. Green Chem. 2019, 21, 4025.
doi: 10.1039/C9GC01766F |
|
|
(m) Silva, T. S.; Zeoly, L. A.; Coelho, F. J. Org. Chem. 2020, 85, 5438.
doi: 10.1021/acs.joc.0c00189 |
|
|
(n) Guidotti, B. B.; Silva, T. S. D.; Correia, J. T. M.; Coelho, F. Org. Biomol. Chem. 2020, 18, 7330.
doi: 10.1039/D0OB01714K |
|
| [5] |
(a) Jana, R.; Pathak, T. P.; Jensen, K. H.; Sigman, M. S. Org. Lett. 2012, 14, 4074.
doi: 10.1021/ol3016989 |
|
(b) Correia, J. T. M.; List, B.; Coelho, F. Angew. Chem., Int. Ed. 2017, 56, 7967.
doi: 10.1002/anie.201700513 |
|
|
(c) Chen, H.; Zhu, L.; Zhong, K.; Yue, X.; Qu, L.-B.; Bai, R.; Lan, Y. Chin. Chem. Lett. 2018, 29, 1237.
doi: 10.1016/j.cclet.2018.03.018 |
|
|
(d) Yang, P.-J.; Qi, L.; Liu, Z.; Yang, G.; Chai, Z. J. Am. Chem. Soc. 2018, 140, 17211.
doi: 10.1021/jacs.8b10217 |
|
|
(e) Yang, L.; Pu, X.; Niu, D.; Fu, Z.; Zhang, X. Org. Lett. 2019, 21, 8553.
doi: 10.1021/acs.orglett.9b03032 |
|
|
(f) Li, K.; Li, C. Org. Lett. 2020, 22, 9456.
doi: 10.1021/acs.orglett.0c03383 |
|
| [6] |
(a) Hartwig, J. F.; Stanley, L. M. Acc. Chem. Res. 2010, 43, 1461.
doi: 10.1021/ar100047x |
|
(b) Qu, J.; Helmchen, G. Acc. Chem. Res. 2017, 50, 2539.
doi: 10.1021/acs.accounts.7b00300 |
|
|
(c) Deng, Y.; Yang, W.; Yang, X.; Yang, D. Chin. J. Org. Chem. 2017, 37, 3039. (in Chinese)
doi: 10.6023/cjoc201704034 |
|
|
(邓颖颍, 杨文, 杨新, 杨定乔, 有机化学, 2017, 37, 3039.)
doi: 10.6023/cjoc201704034 |
|
|
(d) Cheng, Q.; Tu, H.-F.; Zheng, C.; Qu, J.-P.; Helmchen, G.; You, S.-L. Chem. Rev. 2019, 119, 1855.
doi: 10.1021/acs.chemrev.8b00506 |
|
|
(e) Rössler, S. L.; Petrone, D. A.; Carreira, E. M. Acc. Chem. Res. 2019, 52, 2657.
doi: 10.1021/acs.accounts.9b00209 |
|
|
(f) Tian, F.; Zhang, J.; Yang, W.; Deng, W. Chin. J. Org. Chem. 2020, 40, 3262. (in Chinese)
doi: 10.6023/cjoc202005008 |
|
|
(田飞, 张键, 杨武林, 邓卫平, 有机化学, 2020, 40, 3262.)
doi: 10.6023/cjoc202005008 |
|
| [7] |
(a) Janssen, J. P.; Helmchen, G. Tetrahedron Lett. 1997, 38, 8025.
doi: 10.1016/S0040-4039(97)10220-9 |
|
(b) Alexakis, A.; Polet, D. Org. Lett. 2004, 6, 3529.
doi: 10.1021/ol048607y |
|
|
(c) Streiff, S.; Welter, C.; Schelwies, M.; Lipowsky, G.; Miller, N.; Helmchen, G. Chem. Commun. 2005,2957.
|
|
|
(d) Chen, W.; Hartwig, J. F. J. Am. Chem. Soc. 2013, 135, 2068.
doi: 10.1021/ja311363a |
|
|
(e) Liang, X.; Wei, K.; Yang, Y.-R. Chem. Commun. 2015, 51, 17471.
doi: 10.1039/C5CC07221B |
|
|
(f) Jiang, X.; Boehm, P.; Hartwig, J. F. J. Am. Chem. Soc. 2018, 140, 1239.
doi: 10.1021/jacs.7b12824 |
|
|
(g) Huo, X.; Zhang, J.; Fu, J.; He, R.; Zhang, W. J. Am. Chem. Soc. 2018, 140, 2080.
doi: 10.1021/jacs.8b00187 |
|
|
(h) Sempere, Y.; Carreira, E. M. Angew. Chem., Int. Ed. 2018, 57, 7654.
doi: 10.1002/anie.v57.26 |
|
| [8] |
(a) Ohmura, T.; Hartwig, J. F. J. Am. Chem. Soc. 2002, 124, 15164.
doi: 10.1021/ja028614m |
|
(b) López, F.; Ohmura, T.; Hartwig, J. F. J. Am. Chem. Soc. 2003, 125, 3426.
doi: 10.1021/ja029790y |
|
|
(c) Lipowsky, G.; Helmchen, G. Chem. Commun. 2004,116.
|
|
|
(d) Shu, C.; Leitner, A.; Hartwig, J. F. Angew. Chem., Int. Ed. 2004, 43, 4797.
doi: 10.1002/(ISSN)1521-3773 |
|
|
(e) Tissot-Croset, K.; Polet, D.; Alexakis, A. Angew. Chem., Int. Ed. 2004, 43, 2426.
doi: 10.1002/(ISSN)1521-3773 |
|
|
(f) Fischer, C.; Defieber, C.; Suzuki, T.; Carreira, E. M. J. Am. Chem. Soc. 2004, 126, 1628.
doi: 10.1021/ja0390707 |
|
|
(g) Roggen, M.; Carreira, E. M. J. Am. Chem. Soc. 2010, 132, 11917.
doi: 10.1021/ja105271z |
|
|
(h) Stanley, L. M.; Bai, C.; Ueda, M.; Hartwig, J. F. J. Am. Chem. Soc. 2010, 132, 8918.
doi: 10.1021/ja103779e |
|
|
(i) Roggen, M.; Carreira, E. M. Angew. Chem., Int. Ed. 2011, 50, 5568.
doi: 10.1002/anie.201007716 |
|
| [9] |
(a) Liu, W.-B.; He, H.; Dai, L.-X.; You, S.-L. Org. Lett. 2008, 10, 1815.
doi: 10.1021/ol800409d |
|
(b) Wu, Q.-F.; He, H.; Liu, W.-B.; You, S.-L. J. Am. Chem. Soc. 2010, 132, 11418.
doi: 10.1021/ja105111n |
|
|
(c) Huang, L.; Dai, L.-X.; You, S.-L. J. Am. Chem. Soc. 2016, 138, 5793.
doi: 10.1021/jacs.6b02678 |
|
|
(d) Huang, L.; Cai, Y.; Zhang, H.-J.; Dai, L.-X.; You, S.-L. CCS Chem. 2019, 1, 106.
|
|
|
(e) Zhang, J.; Gao, Y.-S.; Gu, B.-M.; Yang, W.-L.; Tian, B.-X.; Deng, W.-P. ACS Catal. 2021, 11, 3810.
doi: 10.1021/acscatal.1c00081 |
|
|
(f) Jiang, R.; Ding, L.; Zheng, C.; You, S.-L. Science 2021, 371, 380.
doi: 10.1126/science.abd6095 |
|
|
(g) Jiang, R.; Zheng, C.; You, S.-L. Chin. Sci. Bull. doi: 10.1360/TB-2021-0096 (in Chinese)
|
|
|
(蒋茹, 郑超, 游书力, 科学通报, doi: 10.1360/TB-2021-0096.)
|
|
| [10] |
(a) Zhuo, C.-X.; Cheng, Q.; Liu, W.-B.; Zhao, Q.; You, S.-L. Angew. Chem., Int. Ed. 2015, 54, 8475.
doi: 10.1002/anie.201502259 |
|
(b) Huang, L.; Cai, Y.; Zheng, C.; Dai, L.-X.; You, S.-L. Angew. Chem., Int. Ed. 2017, 56, 10545.
doi: 10.1002/anie.v56.35 |
|
| [11] |
(a) Wu, Q.-F.; Liu, W.-B.; Zhuo, C.-X.; Rong, Z.-Q.; Ye, K.-Y.; You, S.-L. Angew. Chem., Int. Ed. 2011, 50, 4455.
doi: 10.1002/anie.201100206 |
|
(b) Nemoto, T.; Ishige, Y.; Yoshida, M.; Kohno, Y.; Kanematsu, M.; Hamada, Y. Org. Lett. 2010, 12, 5020.
doi: 10.1021/ol102190s |
|
|
(c) Yoshida, M.; Nemoto, T.; Zhao, Z.; Ishige, Y.; Hamada, Y. Tetrahedron: Asymmetry 2012, 23, 859.
doi: 10.1016/j.tetasy.2012.05.026 |
|
|
(d) Xu, Q.-L.; Dai, L.-X.; You, S.-L. Org. Lett. 2012, 14, 2579.
doi: 10.1021/ol3008793 |
|
|
(e) Cheng, Q.; Wang, Y.; You, S.-L. Angew. Chem., Int. Ed. 2016, 55, 3496.
doi: 10.1002/anie.201511519 |
|
| [12] |
For a book: You, S.-L;. Asymmetric Dearomatization Reactions, Wiley-VCH, Weinheim, Germany, 2016.
|
|
For reviews: (a) Ding, Q.; Zhou, X.; Fan, R;. Org. Biomol. Chem. 2014, 12, 4807.
doi: 10.1039/C4OB00371C |
|
|
(b) Zhuo, C.-X.; Zheng, C.; You, S.-L. Acc. Chem. Res. 2014, 47, 2558.
doi: 10.1021/ar500167f |
|
|
(c) Wu, W.-T.; Zhang, L.; You, S.-L. Chem. Soc. Rev. 2016, 45, 1570.
doi: 10.1039/C5CS00356C |
|
|
(d) Zheng, C.; You, S.-L. Chem 2016, 1, 830.
doi: 10.1016/j.chempr.2016.11.005 |
|
|
(e) Wu, W.-T.; Zhang, L.; You, S.-L. Acta Chim. Sinica 2017, 75, 419. (in Chinese)
doi: 10.6023/A17020049 |
|
|
(吴文挺, 张立明, 游书力, 化学学报, 2017, 75, 419.)
doi: 10.6023/A17020049 |
|
|
(f) Zhu, M.; Zhang, X.; You, S.-L. Chem. J. Chin. Univ. 2020, 41, 1407. (in Chinese)
|
|
|
(朱敏, 张霄, 游书力, 高等学校化学学报, 2020, 41, 1407.)
|
|
| [13] |
During the preparation of our manuscript, Zeng and co-workers reported an iridium catalyzed asymmetric allylic substitution reaction of indolizine derivatives, see: Lu, J.; Wang, M.; Xu, R.; Sun, H.; Zheng, X.; Zhong, G.; Zeng, X.. Asian J. Org. Chem. 2021, 10, 1500.
doi: 10.1002/ajoc.v10.6 |
| [14] |
Defieber, C.; Ariger, M. A.; Moriel, P.; Carreira, E. M. Angew. Chem., Int. Ed. 2007, 46, 3139.
doi: 10.1002/(ISSN)1521-3773 |
| [15] |
Rössler, S. L.; Krautwald, S.; Carreira, E. M. J. Am. Chem. Soc. 2017, 139, 3603.
doi: 10.1021/jacs.6b12421 |
| [1] | 黄涎廷, 韩洪亮, 肖婧, 王帆, 柳忠全. I2O5/KSCN介导的炔烃碘硫氰化反应[J]. 化学学报, 2024, 82(1): 5-8. |
| [2] | 韩叶强, 史炳锋. 钯(II)催化不对称C(sp3)—H键官能团化研究进展★[J]. 化学学报, 2023, 81(11): 1522-1540. |
| [3] | 何倩, 李杰, 喻思佳, 吴东坪, 叶剑良, 黄培强. 铱催化叔酰胺与呋喃硅醚间的类插烯Aldol缩合反应: γ-亚苄基-丁烯酸内酯的合成★[J]. 化学学报, 2023, 81(10): 1265-1270. |
| [4] | 田小茂, 林悦群, 朱菡, 黄超, 朱必学. 手性单Schiff碱大环对青霉胺对映体识别研究[J]. 化学学报, 2023, 81(1): 20-28. |
| [5] | 赵庆如, 蒋茹, 游书力. 铱催化串联不对称烯丙基取代/双键异构化构建轴手性化合物[J]. 化学学报, 2021, 79(9): 1107-1112. |
| [6] | 邓卓基, 欧阳溢凡, 敖运林, 蔡倩. 铜催化不对称去对称化分子内烯基C—N偶联反应[J]. 化学学报, 2021, 79(5): 649-652. |
| [7] | 杨妲, 张龙力, 刘欢, 杨朝合. 双功能配体修饰的Ir催化剂在“氢甲酰化-缩醛化”串联反应中的共催化作用[J]. 化学学报, 2021, 79(5): 658-662. |
| [8] | 周波, 梁仁校, 曹中艳, 周平海, 贾义霞. 钯催化吡咯环内共轭双键的Heck反应[J]. 化学学报, 2021, 79(2): 176-179. |
| [9] | 张荣华, 许冰, 张展鸣, 张俊良. Ming-Phos/铜催化的亚甲胺叶立德与硝基烯烃的不对称[3+2]环加成反应[J]. 化学学报, 2020, 78(3): 245-249. |
| [10] | 张洪浩, 俞寿云. 过渡金属与光氧化还原协同催化的烯丙基取代反应的研究进展[J]. 化学学报, 2019, 77(9): 832-840. |
| [11] | 张毛毛, 骆元元, 陆良秋, 肖文精. 过渡金属与有机小分子协同催化的不对称烯丙基取代反应研究进展[J]. 化学学报, 2018, 76(11): 838-849. |
| [12] | 李娅琼, 黄志真. 过渡金属催化和有机小分子参与的α,β-不饱和酮与烯丙醇的Morita-Baylis-Hillman反应[J]. 化学学报, 2017, 75(3): 280-283. |
| [13] | 张子競, 陶忠林, 阿拉法特·阿地力, 龚流柱. 钯配合物和手性磷酸连续催化的烯丙醇和醛的不对称羰基烯丙基化反应[J]. 化学学报, 2017, 75(12): 1196-1201. |
| [14] | 周容, 肖微, 尹祥, 詹固, 陈应春. 环状烯酮与环状1-氮杂二烯的非对映及对映选择性[4+2]环加成反应[J]. 化学学报, 2014, 72(7): 862-866. |
| [15] | 刘湘,张宝立,夏咏梅,许建和. 用芹菜茎催化对映选择性还原芳香酮[J]. 化学学报, 2009, 67(13): 1492-1496. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||