化学学报 ›› 2023, Vol. 81 ›› Issue (6): 588-594.DOI: 10.6023/A23040153 上一篇 下一篇
所属专题: 庆祝《化学学报》创刊90周年合辑
研究论文
投稿日期:2023-04-20
发布日期:2023-05-17
作者简介:基金资助:
Yinfeng Wanga,b, Meng Lia,b( ), Chuanfeng Chena,b(
), Chuanfeng Chena,b( )
)
Received:2023-04-20
Published:2023-05-17
Contact:
* E-mail: limeng@iccas.ac.cn; cchen@iccas.ac.cn
About author:Supported by:文章分享

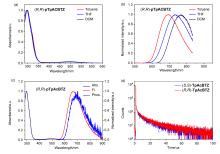
手性三蝶烯并吖啶(TpAc)具有独特的三维结构、同共轭效应和两个分离的反应位点. 采用手性给体-受体(D*-A)共聚的设计策略, 以TpAc作为电子给体与强电子受体4,7-二溴苯并[c][1,2,5]噻二唑(BTZ)直接偶联聚合, 得到了非共轭型手性红光热激活延迟荧光(TADF)聚合物(R,R)-/(S,S)-pTpAcBTZ. 所得聚合物最高占据分子轨道(HOMO)和最低未占分子轨道(LUMO)有效分离并获得小的ΔEST (0.08 eV), 具有显著的红光TADF性质(λtoluene=663 nm). 同时聚合物显示出镜像的红光圆偏振发光(CPL)信号, 其|glum|值约为1.4×10-3. 通过溶液旋涂法制备了红光发光二极管(OLEDs), 所得器件最大发射峰为658 nm, 其在1.0 cd/m2的亮度下开启电压为3.6 V, 最大外量子效率为2.0%. 该非共轭型手性聚合物的设计和红光TADF性能有利于促进手性发光材料及电致红光器件等相关研究领域的发展.
王银凤, 李猛, 陈传峰. 基于手性三蝶烯的红光热激活延迟荧光聚合物及其有机发光二极管研究★[J]. 化学学报, 2023, 81(6): 588-594.
Yinfeng Wang, Meng Li, Chuanfeng Chen. Chiral Triptycene-Based Red Thermally Activated Delayed Fluorescence Polymers and Their Organic Light-Emitting Diodes★[J]. Acta Chimica Sinica, 2023, 81(6): 588-594.



| Devicea | VTb/V | λELc/nm | EQEmaxd/% | CEmaxe/(cd•A-1) | PEmaxf/(lm•W-1) | Lmaxg/(cd•m-2) |
|---|---|---|---|---|---|---|
| A | 3.6 | 658 | 2.0 | 1.1 | 0.8 | 1058 |
| B | 3.5 | 658 | 1.8 | 1.0 | 0.8 | 949 |
| Devicea | VTb/V | λELc/nm | EQEmaxd/% | CEmaxe/(cd•A-1) | PEmaxf/(lm•W-1) | Lmaxg/(cd•m-2) |
|---|---|---|---|---|---|---|
| A | 3.6 | 658 | 2.0 | 1.1 | 0.8 | 1058 |
| B | 3.5 | 658 | 1.8 | 1.0 | 0.8 | 949 |
| [1] |
(a) Farshchi, R.; Ramsteiner, M.; Herfort, J.; Tahraoui, A.; Grahn, H. T. Appl. Phys. Lett. 2011, 98, 162508.
|
|
(b) Kim, D.-Y. J. Korean Phys. Soc. 2006, 49, 505.
|
|
|
(c) Feng, H.; Li, Q.; Wan, W.; Song, J.-H.; Gong, Q.; Brongersma, M. L.; Li, Y. ACS Photonics 2019, 6, 2910.
doi: 10.1021/acsphotonics.9b01017 |
|
|
(d) Nishizawa, N.; Hamada, A.; Takahashi, K.; Kuchimaru, T.; Munekata, H. Jpn. J. Appl. Phys. 2020, 59, SEEG03.
|
|
|
(e) Zhang, X. G.; Yu, Q.; Jiang, W. X.; Sun, Y. L.; Bai, L.; Wang, Q.; Qiu, C.-W.; Cui, T. J. Adv. Sci. 2020, 7, 1903382.
|
|
|
(f) Zhang, L.; Zhao, W.-L.; Li, M.; Lü, H.-Y.; Chen, C.-F. Acta Chim. Sinica 2020, 78, 1030. (in Chinese)
doi: 10.6023/A20060243 |
|
|
(张亮, 赵文龙, 李猛, 吕海燕, 陈传峰, 化学学报, 2020, 78, 1030.)
doi: 10.6023/A20060243 |
|
| [2] |
Grell, M.; Oda, M.; Whitehead, K. S.; Asimakis, A.; Neher, D.; Bradley, D. D. C. Adv. Mater. 2001, 13, 577.
doi: 10.1002/(ISSN)1521-4095 |
| [3] |
Li, M.; Lin, W.-B.; Fang, L.; Chen, C.-F. Acta Chim. Sinica 2017, 75, 1150. (in Chinese)
doi: 10.6023/A17090440 |
|
(李猛, 林伟彬, 房蕾, 陈传峰, 化学学报, 2017, 75, 1150.)
doi: 10.6023/A17090440 |
|
| [4] |
(a) Zhang, D.-W.; Li, M.; Chen, C.-F. Chem. Soc. Rev. 2020, 49, 1331.
doi: 10.1039/C9CS00680J |
|
(b) Gong, Z.-L.; Zhu, X.; Zhou, Z.; Zhang, S.-W.; Yang, D.; Zhao, B.; Zhang, Y.-P.; Deng, J.; Cheng, Y.; Zheng, Y.-X.; Zang, S.-Q.; Kuang, H.; Duan, P.; Yuan, M.; Chen, C.-F.; Zhao, Y. S.; Zhong, Y.-W.; Tang, B. Z.; Liu, M. Sci. China: Chem. 2021, 64, 2060.
|
|
|
(c) Wang, M.; Zhao, C.-H. Chem. Rec. 2022, 22, e202100199.
|
|
| [5] |
(a) Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Nature 2012, 492, 234.
doi: 10.1038/nature11687 |
|
(b) Zhang, L.; Wang, Y.-F.; Li, M.; Gao, Q.-Y.; Chen, C.-F. Chin. Chem. Lett. 2021, 32, 740.
doi: 10.1016/j.cclet.2020.07.041 |
|
| [6] |
Li, M.; Li, S.-H.; Zhang, D.; Cai, M.; Duan, L.; Fung, M.-K.; Chen, C.-F. Angew. Chem., Int. Ed. 2018, 57, 2889.
doi: 10.1002/anie.201800198 |
| [7] |
(a) Zhang, Y.; Zhang, X.; Zhang, H.; Xiao, Y.; Quan, Y.; Ye, S.; Cheng, Y. J. Phys. Chem. C 2019, 123, 24746.
doi: 10.1021/acs.jpcc.9b07414 |
|
(b) Sun, S.; Wang, J.; Chen, L.; Chen, R.; Jin, J.; Chen, C.; Chen, S.; Xie, G.; Zheng, C.; Huang, W. J. Mater. Chem. C 2019, 7, 14511.
doi: 10.1039/C9TC04941J |
|
|
(c) Wu, Z.-G.; Han, H.-B.; Yan, Z.-P.; Luo, X.-F.; Wang, Y.; Zheng, Y.-X.; Zuo, J.-L.; Pan, Y. Adv. Mater. 2019, 1900524.
|
|
|
(d) Li, M.; Wang, Y.-F.; Zhang, D.; Duan, L.; Chen, C.-F. Angew. Chem., Int. Ed. 2020, 59, 3500.
doi: 10.1002/anie.v59.9 |
|
|
(e) Yang, S.-Y.; Wang, Y.-K.; Peng, C.-C.; Wu, Z.-G.; Yuan, S.; Yu, Y.-J.; Li, H.; Wang, T.-T.; Li, H.-C.; Zheng, Y.-X.; Jiang, Z.-Q.; Liao, L.-S. J. Am. Chem. Soc. 2020, 142, 17756.
doi: 10.1021/jacs.0c08980 |
|
|
(f) Xu, Y.; Wang, Q.; Cai, X.; Li, C.; Wang, Y. Adv. Mater. 2021, 33, 2100652.
|
|
|
(g) Ni, F.; Huang, C.-W.; Tang, Y.; Chen, Z.; Wu, Y.; Xia, S.; Cao, X.; Hsu, J.-H.; Lee, W.-K.; Zheng, K.; Huang, Z.; Wu, C.-C.; Yang, C. Mater. Horiz. 2021, 8, 547.
doi: 10.1039/D0MH01521K |
|
|
(h) Yan, Z.-P.; Liu, T.-T.; Wu, R.; Liang, X.; Li, Z.-Q.; Zhou, L.; Zheng, Y.-X.; Zuo, J.-L. Adv. Funct. Mater. 2021, 31, 2103875.
|
|
|
(i) Tan, K.-K.; Zhang, D.-W.; Zhao, W.-L.; Li, M.; Chen, C.-F. Chem. Eng. J. 2023, 462, 142123.
|
|
|
(j) Zhao, W.-L.; Wang, Y.-F.; Wan, S.-P.; Lu, H.-Y.; Li, M.; Chen, C.-F. CCS Chem. 2022, 4, 3540.
doi: 10.31635/ccschem.021.202101509 |
|
|
(k) Liang, Z.-P.; Tang, R.; Qiu, Y.-C.; Wang, Y.; Lu, H.; Wu, Z.-G. Acta Chim. Sinica 2021, 79, 1401. (in Chinese)
doi: 10.6023/A21070355 |
|
|
(梁志鹏, 唐瑞, 邱雨晨, 王阳, 陆洪彬, 吴正光, 化学学报, 2021, 79, 1401.)
doi: 10.6023/A21070355 |
|
| [8] |
Zou, Y.; Gong, S.; Xie, G.; Yang, C. Adv. Optical Mater. 2018, 6, 1800568.
|
| [9] |
(a) Shao, S.; Ding, J.; Wang, L. Chin. J. Appl. Chem. 2018, 35, 993. (in Chinese)
|
|
(邵世洋, 丁军桥, 王利祥, 应用化学, 2018, 35, 993.)
doi: 10.11944/j.issn.1000-0518.2018.09.180202 |
|
|
(b) Wei, Q.; Ge, Z.; Voit, B. Micromol. Rapid. Commun. 2019, 40, 1800570.
|
|
| [10] |
(a) Chen, C.-F.; Han, Y. Acc. Chem. Res. 2018, 51, 2093.
doi: 10.1021/acs.accounts.8b00268 |
|
(b) He, Y.; Yang, X.; Qi, M.; Chen, C.-F. Chin. Chem. Lett. 2021, 6, 2043.
|
|
| [11] |
(a) Wang, Y.-F.; Li, M.; Teng, J.-M.; Zhou, H.-Y.; Chen, C.-F. Adv. Funct. Mater. 2021, 31, 2106418.
|
|
(b) Wang, Y.-F.; Li, M.; Teng, J.-M.; Zhou, H.-Y.; Zhao, W.-L.; Chen, C.-F. Angew. Chem., Int. Ed. 2021, 60, 23619.
doi: 10.1002/anie.v60.44 |
|
| [12] |
Naveen, K. R.; Prabhu, C. P. K.; Braveenth, R.; Kwon, J. H. Chem.- Eur. J. 2022, 28, e202103532.
|
| [13] |
(a) Frédéric, L.; Desmarchelier, A.; Favereau, L.; Pieters, G. Adv. Funct. Mater. 2021, 31, 2010281.
|
|
(b) Li, M.; Chen, C.-F. Org. Chem. Front. 2022, 9, 6441.
doi: 10.1039/D2QO01383E |
|
|
(c) Li, M.; Wang, Y.-F.; Zhang, D.-W.; Zhang, D.; Hu, Z.-Q.; Duan, L.; Chen, C.-F. Sci. China Mater. 2021, 64, 899.
doi: 10.1007/s40843-020-1496-7 |
|
|
(d) Yang, W.; Li, N.; Miao, J.; Zhan, L.; Gong, S.; Huang, Z.; Yang, C. CCS Chem. 2022, 4, 3463.
doi: 10.31635/ccschem.022.202101661 |
|
|
(e) Dong, X.; Shen, S.; Qin, Y.; Hu, X.; Gao, H.; Liu, G.; Gao, T.; Pang, Z.; Wang, P.; Wang, Y. Chin. Chem. Lett. 2023, doi: 10.1016/j.cclet.2023.108311.
doi: 10.1016/j.cclet.2023.108311 |
| [1] | 易敬霖, 陈茂. 三氟氯乙烯与甲基异丙烯基醚的光诱导共聚反应★[J]. 化学学报, 2024, 82(2): 126-131. |
| [2] | 李雅宁, 王晓艳, 唐勇. 自由基聚合的立体选择性调控★[J]. 化学学报, 2024, 82(2): 213-225. |
| [3] | 魏颖, 王家成, 李玥, 汪涛, 马述威, 解令海. 碳碳键链接的二维共价有机框架研究进展[J]. 化学学报, 2024, 82(1): 75-102. |
| [4] | 田野, 司端惠, 高水英, 曹荣. 苯二甲酸衍生物修饰聚合物的超长有机室温磷光★[J]. 化学学报, 2023, 81(9): 1129-1134. |
| [5] | 万义, 何江华, 张越涛. Lewis酸碱对催化极性烯烃单体精准聚合的研究进展★[J]. 化学学报, 2023, 81(9): 1215-1230. |
| [6] | 贾凌轩, 詹泽庞, 贺紫晗, 狄重安, 朱道本. 面向神经电子接口器件的有机材料进展与展望★[J]. 化学学报, 2023, 81(9): 1175-1186. |
| [7] | 葛凤洁, 张开志, 曹清鹏, 徐慧, 周涛, 张文浩, 班鑫鑫, 张晓波, 李娜, 朱鹏. 柔性芴基嵌段型延迟荧光二聚体的设计、合成及电致发光性能[J]. 化学学报, 2023, 81(9): 1157-1166. |
| [8] | 于乐飞, 姚兴奇, 王剑波. 重氮化合物在高分子合成化学中的应用进展★[J]. 化学学报, 2023, 81(8): 1015-1029. |
| [9] | 苑志祥, 张浩, 胡思伽, 张波涛, 张建军, 崔光磊. 离子聚合原位固态化构建高安全锂电池固态聚合物电解质的研究进展★[J]. 化学学报, 2023, 81(8): 1064-1080. |
| [10] | 孙博, 琚雯雯, 王涛, 孙晓军, 赵婷, 卢晓梅, 陆峰, 范曲立. 高分散共轭聚合物-金属有机框架纳米立方体的制备及抗肿瘤应用[J]. 化学学报, 2023, 81(7): 757-762. |
| [11] | 郭斌, 王铭轩, 张荻琴, 孙敏远, 毕勇, 赵榆霞. 用于制备全息光波导的光致聚合物的研究进展[J]. 化学学报, 2023, 81(4): 393-405. |
| [12] | 张慧颖, 于淑艳, 李从举. 高分子聚合物基碳纳米膜的电催化降解污水性能及机理[J]. 化学学报, 2023, 81(4): 420-430. |
| [13] | 张少秦, 李美清, 周中军, 曲泽星. 多共振热激活延迟荧光过程的理论研究[J]. 化学学报, 2023, 81(2): 124-130. |
| [14] | 李东旭, 徐翔, 宋佳鸽, 梁松挺, 付予昂, 路新慧, 邹应萍. 轮烷结构优化聚合物太阳能电池光伏性能★[J]. 化学学报, 2023, 81(11): 1500-1507. |
| [15] | 溥旭, 李泽娟, 石隽秋, 朱云卿, 杜建忠. 器官靶向的聚合物核酸载体研究进展★[J]. 化学学报, 2023, 81(10): 1438-1446. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||