化学学报 ›› 2024, Vol. 82 ›› Issue (2): 126-131.DOI: 10.6023/A23080387 上一篇 下一篇
所属专题: 庆祝《化学学报》创刊90周年合辑; 有机氟化学合集
研究论文
投稿日期:2023-08-21
发布日期:2023-10-05
作者简介:基金资助:Received:2023-08-21
Published:2023-10-05
Contact:
E-mail: About author:Supported by:文章分享

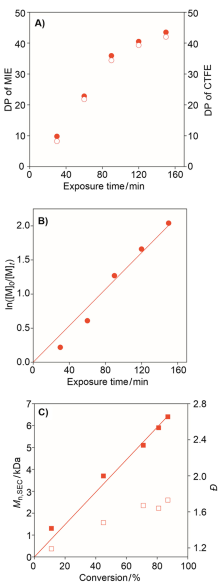
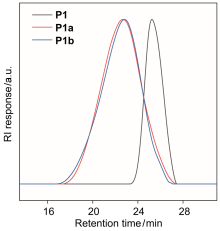
氟聚合物综合性能优异, 在诸多领域发挥了重要作用. 三氟氯乙烯(chlorotrifluoroethylene, CTFE)与乙烯基醚共聚物被用于高性能涂料, 但其较低的玻璃化转变温度对应用场景带来了局限. 在本工作中, 发展了CTFE与甲基异丙烯基醚(methyl isopropenyl ether, MIE)的光照自由基共聚反应, 在室温常压条件下合成了全新化学结构的氟烯烃与烯基醚共聚物. 聚合反应过程符合一级动力学与交替共聚特征, 通过控制链转移剂用量与MIE转化率可合成不同分子量的共聚物, 表明该聚合反应具备一定的可控性, 但共聚物分子量分布较宽、链末端保真度有限. 在此基础上, 本工作首次揭示了CTFE-MIE共聚物比CTFE-乙烯基乙醚共聚物的玻璃化转变温度提高了近50 ℃, 有助于进一步开发高性能氟聚合物材料.
易敬霖, 陈茂. 三氟氯乙烯与甲基异丙烯基醚的光诱导共聚反应★[J]. 化学学报, 2024, 82(2): 126-131.
Jinglin Yi, Mao Chen. Photo-Induced Copolymerization of Chlorotrifluoroethylene and Methyl Isopropenyl Ether★[J]. Acta Chimica Sinica, 2024, 82(2): 126-131.




| Entry | CTA | Light source | Conv. (MIE)b/% | Mn,GPCc/kDa | Ðc |
|---|---|---|---|---|---|
| 1 | 1a | blue | 85 | 10.7 | 2.11 |
| 2 | 1b | blue | 80 | 9.9 | 1.96 |
| 3 | 1c | blue | 20 | 4.4 | 2.13 |
| 4 | 2a | blue | 90 | 8.3 | 1.96 |
| 5 | 2b | blue | 85 | 5.6 | 1.64 |
| 6 | 2c | blue | 76 | 5.9 | 1.57 |
| 7 | 3c | blue | <5 | — | — |
| 8 | 4c | blue | 83 | 8.6 | 2.02 |
| 9 | 5c | blue | <5 | — | — |
| 10 | 2c | — | 0 | — | — |
| 11 | 2c | purple | 79 | 5.3 | 1.64 |
| 12 | 2c | white | 52 | 3.4 | 1.53 |
| 13d | 2c | white | <5 | — | — |
| Entry | CTA | Light source | Conv. (MIE)b/% | Mn,GPCc/kDa | Ðc |
|---|---|---|---|---|---|
| 1 | 1a | blue | 85 | 10.7 | 2.11 |
| 2 | 1b | blue | 80 | 9.9 | 1.96 |
| 3 | 1c | blue | 20 | 4.4 | 2.13 |
| 4 | 2a | blue | 90 | 8.3 | 1.96 |
| 5 | 2b | blue | 85 | 5.6 | 1.64 |
| 6 | 2c | blue | 76 | 5.9 | 1.57 |
| 7 | 3c | blue | <5 | — | — |
| 8 | 4c | blue | 83 | 8.6 | 2.02 |
| 9 | 5c | blue | <5 | — | — |
| 10 | 2c | — | 0 | — | — |
| 11 | 2c | purple | 79 | 5.3 | 1.64 |
| 12 | 2c | white | 52 | 3.4 | 1.53 |
| 13d | 2c | white | <5 | — | — |
| Entry | [CTFE]/[MIE]/[CTA] | Conv. (MIE)b/% | Mn,GPCc/kDa | Mn,MALSd/kDa | Ðc |
|---|---|---|---|---|---|
| 1 | 400/200/1 | >99 | 19.1 | 1.77 | |
| 2 | 400/300/1 | 83 | 16.2 | 1.75 | |
| 3 | 800/400/1 | >99 | 24.8 | 87.5 | 1.85 |
| 4 | 1000/500/1 | >99 | 34.8 | 212.8 | 1.90 |
| Entry | [CTFE]/[MIE]/[CTA] | Conv. (MIE)b/% | Mn,GPCc/kDa | Mn,MALSd/kDa | Ðc |
|---|---|---|---|---|---|
| 1 | 400/200/1 | >99 | 19.1 | 1.77 | |
| 2 | 400/300/1 | 83 | 16.2 | 1.75 | |
| 3 | 800/400/1 | >99 | 24.8 | 87.5 | 1.85 |
| 4 | 1000/500/1 | >99 | 34.8 | 212.8 | 1.90 |


| [1] |
Améduri, B. Chem. Eur. J. 2018, 24, 18830.
doi: 10.1002/chem.v24.71 |
| [2] |
Scheirs, J. Modern fluoropolymers: High performance polymers for diverse applications, Wiley, New York, 1997.
|
| [3] |
Lv, J.; Cheng, Y. Chem. Soc. Rev. 2021, 50, 5435.
doi: 10.1039/D0CS00258E |
| [4] |
Yao, W.; Li, Y.; Huang, X. Polymer 2014, 55, 6197.
doi: 10.1016/j.polymer.2014.09.036 |
| [5] |
Xu, J.; Lv, J.; Zhuang, Q.; Yang, Z.; Cao, Z.; Xu, L.; Pei, P.; Wang, C.; Wu, H.; Dong, Z.; Chao, Y.; Wang, C.; Yang, K.; Peng, R.; Cheng, Y.; Liu, Z. Nat. Nanotechnol. 2020, 15, 1043.
doi: 10.1038/s41565-020-00781-4 |
| [6] |
Chen, Y.; Zhang, S.; Feng, C.; Zhang, Y.; Li, Q.; Li, W.; Huang, X. Chin. J. Chem. 2009, 27, 2261.
doi: 10.1002/cjoc.v27:11 |
| [7] |
Puts, G. J.; Crouse, P.; Améduri, B. Chem. Rev. 2019, 119, 1763.
doi: 10.1021/acs.chemrev.8b00458 |
| [8] |
Ebnesajjad, S. Introduction to fluoropolymers: Materials, technology, and applications, Elsevier, Waltham, 2013.
|
| [9] |
Améduri, B. Macromolecules 2010, 43, 10163.
doi: 10.1021/ma1019297 |
| [10] |
Hougham, G.; Cassidy, P.; Johns, K.; Davidson, J. Fluoropolymers: Synthesis and applications, Plenum, New York, 1999.
|
| [11] |
Gong, H.; Zhang, Y.; Cheng, Y.; Lei, M.; Zhang, Z. Chinese J. Polym. Sci. 2021, 39, 1110.
doi: 10.1007/s10118-021-2616-x |
| [12] |
Qian, X.; Chen, X.; Zhu, L.; Zhang, Q. M. Science 2023, 380, eadg0902.
doi: 10.1126/science.adg0902 |
| [13] |
Yu, Q.; Wang, J.; Cheng, J.; Zhang, L.; Cheng, Z. Appl. Surf. Sci. 2023, 614, 156199.
doi: 10.1016/j.apsusc.2022.156199 |
| [14] |
Li, J.; Hu, X.; Gao, G.; Ding, S.; Li, H.; Yang, L.; Zhang, Z. J. Mater. Chem. C 2013, 1, 1111.
doi: 10.1039/C2TC00431C |
| [15] |
Tan, S.; Xiong, J.; Zhao, Y.; Liu, J.; Zhang, Z. J. Mater. Chem. C 2018, 6, 4131.
doi: 10.1039/C8TC00781K |
| [16] |
Mihelčič, M.; Slemenik Perše, L.; Šest, E.; Jerman, I.; Giuliani, C.; Di Carlo, G.; Lavorgna, M.; Surca, A. K. Prog. Org. Coat. 2018, 125, 266.
|
| [17] |
Munekata, S. Prog. Org. Coat. 1988, 16, 113.
doi: 10.1016/0033-0655(88)80010-4 |
| [18] |
Boschet, F.; Améduri, B. Chem. Rev. 2013, 114, 927.
doi: 10.1021/cr2002933 |
| [19] |
Scheirs, J.; Burks, S.; Locaspi, A. Trends Polym. Sci. 1995, 3, 74.
|
| [20] |
Améduri, B.; Boutevin, B.; Kostov, G. Prog. Polym. Sci. 2001, 26, 105.
doi: 10.1016/S0079-6700(00)00044-7 |
| [21] |
Vyazovkin, S.; Dranca, I. Thermochim. Acta 2006, 446, 140.
doi: 10.1016/j.tca.2006.04.017 |
| [22] |
Metin, B.; Blum, F. D. J. Chem. Phys. 2006, 125, 054707.
doi: 10.1063/1.2219739 |
| [23] |
An, Z.; Zhu, S.; An, Z. Polym. Chem. 2021, 12, 2357.
doi: 10.1039/D1PY00130B |
| [24] |
Cole, J. P.; Federico, C. R.; Lim, C.-H.; Miyake, G. M. Macromolecules 2019, 52, 747.
doi: 10.1021/acs.macromol.8b02688 |
| [25] |
Discekici, E. H.; Anastasaki, A.; Kaminker, R.; Willenbacher, J.; Truong, N. P.; Fleischmann, C.; Oschmann, B.; Lunn, D. J.; Read de Alaniz, J.; Davis, T. P.; Bates, C. M.; Hawker, C. J. J. Am. Chem. Soc. 2017, 139, 5939.
doi: 10.1021/jacs.7b01694 pmid: 28406296 |
| [26] |
Dadashi-Silab, S.; Matyjaszewski, K. ACS Macro Lett. 2019, 8, 1110.
doi: 10.1021/acsmacrolett.9b00579 |
| [27] |
Wu, C.; Chen, H.; Corrigan, N.; Jung, K.; Kan, X.; Li, Z.; Liu, W.; Xu, J.; Boyer, C. J. Am. Chem. Soc. 2019, 141, 8207.
doi: 10.1021/jacs.9b01096 |
| [28] |
Phommalysack-Lovan, J.; Chu, Y.; Boyer, C.; Xu, J. Chem. Commun. 2018, 54, 6591.
doi: 10.1039/C8CC02783H |
| [29] |
Yamago, S.; Nakamura, Y. Polymer 2013, 54, 981.
doi: 10.1016/j.polymer.2012.11.046 |
| [30] |
Ding, C.; Fan, C.; Jiang, G.; Pan, X.; Zhang, Z.; Zhu, J.; Zhu, X. Macromol. Rapid Commun. 2015, 36, 2181.
doi: 10.1002/marc.v36.24 |
| [31] |
Xiao, Y.; Hu, W.; Xia, Z.; Shi, B.; Lü, C. Chin. J. Chem. 2023, 41, 2604.
doi: 10.1002/cjoc.v41.20 |
| [32] |
Dadashi-Silab, S.; Doran, S.; Yagci, Y. Chem. Rev. 2016, 116, 10212.
doi: 10.1021/acs.chemrev.5b00586 pmid: 26745441 |
| [33] |
Corrigan, N.; Yeow, J.; Judzewitsch, P.; Xu, J.; Boyer, C. Angew. Chem. Int. Ed. 2019, 58, 5170.
doi: 10.1002/anie.v58.16 |
| [34] |
Chen, M.; Zhong, M.; Johnson, J. A. Chem. Rev. 2016, 116, 10167.
doi: 10.1021/acs.chemrev.5b00671 pmid: 26978484 |
| [35] |
Li, S.; Han, G.; Zhang, W. Polym. Chem. 2020, 11, 1830.
doi: 10.1039/D0PY00054J |
| [36] |
Wallentin, C.-J.; Nguyen, J. D.; Finkbeiner, P.; Stephenson, C. R. J. J. Am. Chem. Soc. 2012, 134, 8875.
doi: 10.1021/ja300798k |
| [37] |
Whitfield, R.; Parkatzidis, K.; Rolland, M.; Truong, N. P.; Anastasaki, A. Angew. Chem. Int. Ed. 2019, 58, 13323.
doi: 10.1002/anie.201906471 pmid: 31291503 |
| [38] |
Gao, Q.; Tu, K.; Li, H.; Zhang, L.; Cheng, Z. Sci. China Chem. 2021, 64, 1242.
doi: 10.1007/s11426-021-1002-1 |
| [39] |
Liu, X.; Ni, Y.; Wu, J.; Jiang, H.; Zhang, Z.; Zhang, L.; Cheng, Z.; Zhu, X. Polym. Chem. 2018, 9, 584.
doi: 10.1039/C7PY02008B |
| [40] |
Jiang, K.; Han, S.; Ma, M.; Zhang, L.; Zhao, Y.; Chen, M. J. Am. Chem. Soc. 2020, 142, 7108.
doi: 10.1021/jacs.0c01016 |
| [41] |
Quan, Q.; Ma, M.; Wang, Z.; Gu, Y.; Chen, M. Angew. Chem. Int. Ed. 2021, 60, 20443.
doi: 10.1002/anie.202107066 pmid: 34121303 |
| [42] |
Quan, Q.; Zhao, Y.; Chen, K.; Zhou, H.; Zhou, C.; Chen, M. ACS Catal. 2022, 12, 7269.
doi: 10.1021/acscatal.2c01640 |
| [43] |
Zhao, Y.; Chen, Y.; Zhou, H.; Zhou, Y.; Chen, K.; Gu, Y.; Chen, M. Nat. Synth. 2023, 2, 653.
doi: 10.1038/s44160-023-00284-9 |
| [44] |
Chen, K.; Zhou, Y.; Han, S.; Liu, Y.; Chen, M. Angew. Chem. Int. Ed. 2022, 61, e202116135.
doi: 10.1002/anie.v61.14 |
| [45] |
Ma, M.; Shao, F.; Wen, P.; Chen, K.; Li, J.; Zhou, Y.; Liu, Y.; Jia, M.; Chen, M.; Lin, X. ACS Energy Lett. 2021, 6, 4255.
doi: 10.1021/acsenergylett.1c02036 |
| [46] |
Carnevale, D.; Wormald, P.; Ameduri, B.; Tayouo, R.; Ashbrook, S. E. Macromolecules 2009, 42, 5652.
doi: 10.1021/ma900789t |
| [1] | 李西安, 李孝坤. 基于温度诱导相转变共聚物和导电聚合物的自隔断超级电容器[J]. 化学学报, 2023, 81(5): 511-519. |
| [2] | 汪洋, 向焌钧, 葛从伍, 高希珂. 2,6-薁和3,4-丙撑二氧噻吩共聚物的主链结构调控及性质研究★[J]. 化学学报, 2023, 81(10): 1341-1349. |
| [3] | 李卫华. 桥连对嵌段共聚物自组装的调控[J]. 化学学报, 2021, 79(2): 133-138. |
| [4] | 李荣烨, Khiman Mehul, 盛力, 孙静. 两亲性聚氨基酸三嵌段共聚物构筑pH/溶剂可控多级纳米结构[J]. 化学学报, 2020, 78(11): 1235-1239. |
| [5] | 崔惠娜, 邱枫, 彭娟. 一种基于聚噻吩-聚硒吩全共轭嵌段共聚物的合成及性质研究[J]. 化学学报, 2018, 76(9): 691-700. |
| [6] | 丁妍春, 俞燕蕾, 韦嘉. 不同亲疏水比例的光响应性嵌段共聚物的合成及溶液组装行为研究[J]. 化学学报, 2014, 72(5): 602-608. |
| [7] | 郝莹, 张洋, 何金林, 尚修娟, 张明祖, 倪沛红. 半乳糖胺修饰阳离子型刷形嵌段共聚物的合成与表征[J]. 化学学报, 2014, 72(5): 569-576. |
| [8] | 江昱倩, 徐海华, 赵妮, 彭谦, 帅志刚. 给受共聚物链上与链间极化子的光谱特性[J]. 化学学报, 2014, 72(2): 201-207. |
| [9] | 张广萌, 杨继萍, 陈功. 苯胺四聚体-聚乙二醇-苯胺四聚体嵌段共聚物薄膜的自组装[J]. 化学学报, 2014, 72(1): 83-88. |
| [10] | 王瑞娟, 王毅琳. 阴离子磺酸盐型Gemini表面活性剂与PEO-PPO-PEO嵌段共聚物相互作用的研究[J]. 化学学报, 2014, 72(1): 41-50. |
| [11] | 夏彬凯, 李卫华, 邱枫. 对称AB两嵌段共聚物在均聚物C中的自组装[J]. 化学学报, 2014, 72(1): 30-34. |
| [12] | 杨慧, 彭娟, 邱枫. PI-b-P2VP两嵌段共聚物环状形貌的影响因素研究[J]. 化学学报, 2013, 71(08): 1141-1148. |
| [13] | 王立权, 林嘉平, 张乾. 梳状-线性共聚物自组装的耗散粒子动力学模拟[J]. 化学学报, 2013, 71(06): 913-919. |
| [14] | 潘高翔, 冯泽, 韦嘉, 俞燕蕾. 光/温度双响应三嵌段共聚物的合成及溶液自组装行为[J]. 化学学报, 2013, 71(05): 733-738. |
| [15] | 孙暖暖, 李一鸣, 王东翔, 包木太, 童林娟. Pluronic三嵌段共聚物在油水界面上自组装行为的介观模拟研究[J]. 化学学报, 2013, 71(02): 186-192. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
