化学学报 ›› 2025, Vol. 83 ›› Issue (9): 1013-1017.DOI: 10.6023/A25060202 上一篇 下一篇
研究论文
彭琼慧a, 彭佳a, 蔡迎丽a, 王祖利d, 易荣楠b,*( ), 沈超c,*(
), 沈超c,*( ), 何卫民a,*(
), 何卫民a,*( )
)
投稿日期:2025-06-03
发布日期:2025-07-08
基金资助:
Qionghui Penga, Jia Penga, Yingli Caia, Zuli Wangd, Rongnan Yib,*( ), Chao Shenc,*(
), Chao Shenc,*( ), Weimin Hea,*(
), Weimin Hea,*( )
)
Received:2025-06-03
Published:2025-07-08
Contact:
* E-mail: yrn@hnu.edu.cn;shenchaozju@zjsru.edu.cn;weiminhe@usc.edu.cn
Supported by:文章分享
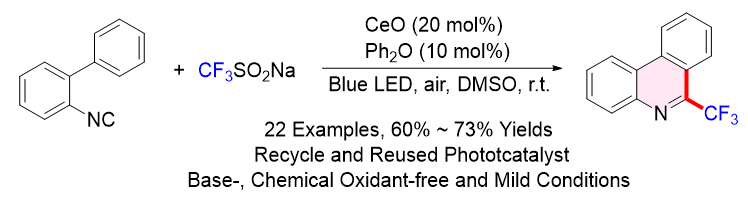
可见光催化可以将光能转化为化学能, 使其在温和条件下实现化学键的断裂与重组, 从而实现一些传统热反应无法实现的化学转化, 具有条件温和、绿色环保以及官能团耐受性高等优点. 可见光催化体系已成功应用于多种复杂分子的合成, 展现出突出的催化合成价值和工业应用潜力. 6-氟烷基菲啶是一种高价值的菲啶衍生物, 具有重要的生物活性和药理价值, 广泛存在于药物和生物活性化合物中. 因此, 6-氟烷基菲啶的高效制备受到了科研工作者的广泛关注. 本文以廉价易得的本征氧化铈半导体作为多相光催化剂, 二苯醚作为均相氧化还原助催化剂, 氟烷基亚磺酸钠作为自由基氟烷基化试剂, 发展了一种无需化学氧化剂和碱添加剂的二苯醚介导氧化铈半多相光催化2-异腈基联苯氟烷基化反应, 以60%~73%的收率合成了22种6-氟烷基菲啶化合物. 该半多相光催化合成方法具有反应条件温和、官能团耐受性好、实验操作简单等优点, 为6-氟烷基菲啶化合物的制备提供了一种绿色可持续的新方法.
彭琼慧, 彭佳, 蔡迎丽, 王祖利, 易荣楠, 沈超, 何卫民. 二苯醚介导氧化铈半多相光催化2-异腈基联苯氟烷基化反应[J]. 化学学报, 2025, 83(9): 1013-1017.
Qionghui Peng, Jia Peng, Yingli Cai, Zuli Wang, Rongnan Yi, Chao Shen, Weimin He. Ph2O-Mediated CeO2-Photocatalyzed Semi-Heterogeneous Fluoroalkylation of 2-Isocyanobiaryls[J]. Acta Chimica Sinica, 2025, 83(9): 1013-1017.
| Entry | Varying from the standard conditions | Yieldb/% |
|---|---|---|
| 1 | None | 73 |
| 2 | g-C3N4, Fe2O3, BiVO4 instead of CeO2 | 36, 45, 32 |
| 3 | CdO, MoO2, ZnO instead of CeO2 | 41, 34, 38 |
| 4 | Without semiconductor photocatalyst | N.R. |
| 5 | NHPI, Cp2Fe, Ph3N instead of Ph2O | 41, 28, 31 |
| 6 | Without redox catalyst | 33 |
| 7 | Purple LED instead of blue LED | 39 |
| 8 | Green LED instead of blue LED | N.R. |
| 9 | White LED instead of blue LED | N.R. |
| 10 | LED (12 W), LED (8 W) instead of LED (10 W) | 72, 63 |
| 11 | Acetone, EtOAc, DMF instead of DMSO | 21, trace, 50 |
| 12 | Without air or light | N.R. |
| Entry | Varying from the standard conditions | Yieldb/% |
|---|---|---|
| 1 | None | 73 |
| 2 | g-C3N4, Fe2O3, BiVO4 instead of CeO2 | 36, 45, 32 |
| 3 | CdO, MoO2, ZnO instead of CeO2 | 41, 34, 38 |
| 4 | Without semiconductor photocatalyst | N.R. |
| 5 | NHPI, Cp2Fe, Ph3N instead of Ph2O | 41, 28, 31 |
| 6 | Without redox catalyst | 33 |
| 7 | Purple LED instead of blue LED | 39 |
| 8 | Green LED instead of blue LED | N.R. |
| 9 | White LED instead of blue LED | N.R. |
| 10 | LED (12 W), LED (8 W) instead of LED (10 W) | 72, 63 |
| 11 | Acetone, EtOAc, DMF instead of DMSO | 21, trace, 50 |
| 12 | Without air or light | N.R. |
| [1] |
(a)
|
|
(b)
|
|
| [2] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(王成强, 冯超, 化学学报, 2024, 82, 160.)
doi: 10.6023/A23080373 |
|
| [3] |
(a)
|
|
(b)
|
|
| [4] |
(a)
|
|
(杨春晖, 陈景超, 李新汉, 孟丽, 王凯民, 孙蔚青, 樊保敏, 化学学报, 2023, 81, 1.)
doi: 10.6023/A22110454 |
|
|
(b)
|
|
|
(易敬霖, 陈茂, 化学学报, 2024, 82, 126.)
doi: 10.6023/A23080387 |
|
| [5] |
|
|
(程步清, 葛丹华, 汪欣, 褚雪强, 有机化学, 2021, 41, 1925.)
doi: 10.6023/cjoc202009035 |
|
| [6] |
|
|
(朱玉溪, 肖婷, 夏冬, 杨文超, 有机化学, 2022, 42, 4067.)
doi: 10.6023/cjoc202208017 |
|
| [7] |
|
| [8] |
(a)
|
|
(b)
|
|
|
(李珊, 路俊欣, 刘杰, 蒋绿齐, 易文斌, 化学学报, 2024, 82, 110.)
doi: 10.6023/A23080386 |
|
|
(c)
|
|
| [9] |
(a)
pmid: 39600199 |
|
(段康慧, 唐俊龙, 伍婉卿, 有机化学, 2023, 43, 826.)
doi: 10.6023/cjoc202211046 pmid: 39600199 |
|
|
(b)
pmid: 39600199 |
|
|
(c)
pmid: 39600199 |
|
|
(d)
pmid: 39600199 |
|
|
(e)
pmid: 39600199 |
|
|
(f)
doi: 10.1039/d4ob01655f pmid: 39600199 |
|
| [10] |
(a)
|
|
(b)
|
|
| [11] |
(a)
|
|
(b)
|
|
| [12] |
(a)
pmid: 25055240 |
|
(b)
doi: 10.1021/ol5018195 pmid: 25055240 |
|
| [13] |
(a)
|
|
(b)
|
|
|
(c)
|
|
| [14] |
(a)
|
|
(陈祥, 欧阳文韬, 李潇, 何卫民, 有机化学, 2023, 43, 4213.)
doi: 10.6023/cjoc202307026 |
|
|
(b)
|
|
|
(辛翠, 蒋俊, 邓紫微, 欧丽娟, 何卫民, 化学学报, 2024, 82, 1109.)
doi: 10.6023/A24100329 |
|
|
(c)
|
|
|
(李康葵, 龙先扬, 黄岳, 祝诗发, 化学学报, 2024, 82, 658.)
doi: 10.6023/A24030090 |
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
|
|
(g)
|
|
|
(h)
|
|
|
(i)
|
|
| [15] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
| [16] |
(a)
pmid: 36880879 |
|
(b)
pmid: 36880879 |
|
|
(c)
doi: 10.1039/c6ob01245k pmid: 36880879 |
|
|
(d)
doi: 10.1039/d3ob00239j pmid: 36880879 |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(戴琳泷, 钟国富, 有机化学, 2023, 43, 2589.)
doi: 10.6023/cjoc202300042 |
|
| [21] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
|
| [22] |
|
| [1] | 闫生荣, 刘文浩, 段芳, 陈明清, 东为富, 陆双龙, 杜明亮. 基于sp2-碳连接具有D-A结构共价有机框架的构筑及其光催化产双氧水性能研究[J]. 化学学报, 2025, 83(7): 685-693. |
| [2] | 崔雨琪, 李军玉, 孙建科. 多孔有机笼用于能源转换及储存的研究进展[J]. 化学学报, 2025, 83(6): 624-638. |
| [3] | 罗雅玲, 庄展洋, 范峰滔, 李灿. Al掺杂SrTiO3的合成调控及其光催化全分解水性能[J]. 化学学报, 2025, 83(6): 608-615. |
| [4] | 李文静, 杨黎燕, 关丽, 张雪娇, 尤静, 沈思语, 赵钰琦, 段琛. 可见光/硫酚催化烯烃C=C双键的氧化裂解反应[J]. 化学学报, 2025, 83(6): 596-601. |
| [5] | 卢一林, 董盛杰, 崔方超, 薄婷婷, 毛卓. 希托夫紫磷烯/SnS2范德华异质结作为直接全解水光催化剂的理论构建[J]. 化学学报, 2025, 83(4): 377-389. |
| [6] | 李国凯, 朱滨锋, 胡涛, 樊瑞峰, 孙蔚青, 和振秀, 陈景超, 樊保敏. 光催化叔胺的脱烷基酰化反应[J]. 化学学报, 2025, 83(3): 199-205. |
| [7] | 胡国伟, 王晓欢, 原志鹏, 新巴雅尔, 乌力吉贺希格. Ag掺杂对铁酸铋系化合物光催化性能的影响[J]. 化学学报, 2025, 83(3): 229-236. |
| [8] | 康健, 石梓煊, 李景梅. 高抗菌性光催化材料的制备及LED光驱动其抗菌性能研究[J]. 化学学报, 2024, 82(9): 962-970. |
| [9] | 张帆帆, 蔡元韬, 陶剑波, 常国菊, 郭欣辰, 郝仕油. Zn, C引入量和煅烧温度对ZnO/C/CeO2光催化还原Cu2+效率的影响[J]. 化学学报, 2024, 82(8): 871-878. |
| [10] | 李康葵, 龙先扬, 黄岳, 祝诗发. 可见光介导炔烃的自由基1,2-官能团化反应新进展[J]. 化学学报, 2024, 82(6): 658-676. |
| [11] | 王国景, 陈永辉, 张秀芹, 张俊笙, 徐俊敏, 王静. 氧空位控制BiVO4晶面异质结的磁性和光电催化性能[J]. 化学学报, 2024, 82(4): 409-415. |
| [12] | 黄广峥, 李坤玮, 罗艳楠, 张强, 潘远龙, 高洪林. 水热后处理构建K掺杂和表面缺陷g-C3N4纳米片促进光催化制氢[J]. 化学学报, 2024, 82(3): 314-322. |
| [13] | 陈健强, 朱钢国, 吴劼. 镍催化氮杂环丙烷的开环偶联反应研究[J]. 化学学报, 2024, 82(2): 190-212. |
| [14] | 辛翠, 蒋俊, 邓紫微, 欧丽娟, 何卫民. 可见光诱导氯化铁催化苯并噁嗪-2-酮与烷烃的脱氢偶联烷基化反应[J]. 化学学报, 2024, 82(11): 1109-1113. |
| [15] | 吴宇晗, 张栋栋, 尹宏宇, 陈正男, 赵文, 匙玉华. “双碳”目标下Janus In2S2X光催化还原CO2的密度泛函理论研究[J]. 化学学报, 2023, 81(9): 1148-1156. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||