有机化学 ›› 2022, Vol. 42 ›› Issue (11): 3658-3667.DOI: 10.6023/cjoc202205054 上一篇 下一篇
所属专题: 有机氟化学虚拟合辑
研究论文
收稿日期:2022-05-31
修回日期:2022-07-14
发布日期:2022-08-25
通讯作者:
肖铁波, 江玉波
基金资助:
Jiazhuang Wang, Liguo Teng, Shaoqi Xiong, Tiebo Xiao( ), Yubo Jiang(
), Yubo Jiang( )
)
Received:2022-05-31
Revised:2022-07-14
Published:2022-08-25
Contact:
Tiebo Xiao, Yubo Jiang
Supported by:文章分享
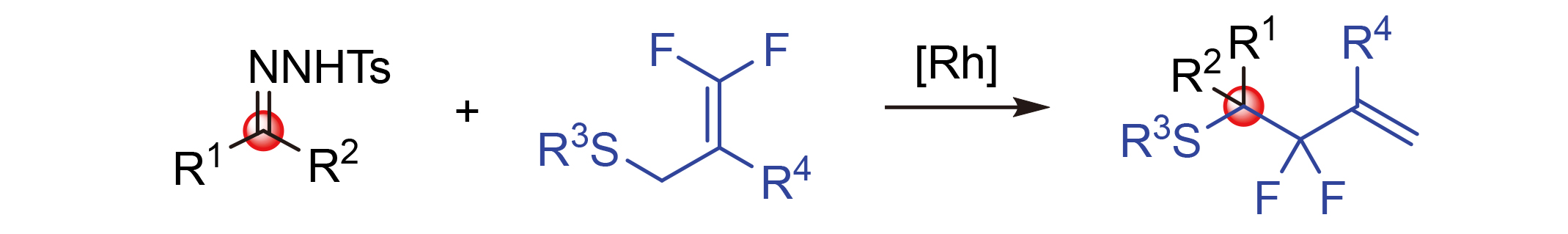
发展了一种铑催化N-磺酰腙和3,3-二氟烯丙基硫化物的重排反应合成偕-二氟烯丙基化合物的高效方法. 该方法具有中等至优异的收率和良好的官能团耐受性, 并通过两步一锅、克级合成和产物的衍生化验证了其实用性.
王家状, 滕丽果, 熊绍棋, 肖铁波, 江玉波. Rh催化N-磺酰腙的偕-二氟烯丙基化反应[J]. 有机化学, 2022, 42(11): 3658-3667.
Jiazhuang Wang, Liguo Teng, Shaoqi Xiong, Tiebo Xiao, Yubo Jiang. Rh-Catalyzed gem-Difluoroallylation of N-Tosylhydrazones[J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3658-3667.
| Entry | Catalyst | Base | Solvent | Temperature/℃ | Yieldb/% |
|---|---|---|---|---|---|
| 1 | [Rh(OAc)2]2 | Cs2CO3 | DCE | 100 | 30 |
| 2 | [Rh(esp)]2 | Cs2CO3 | DCE | 100 | 81 |
| 3 | [Rh(OPiv)2]2 | Cs2CO3 | DCE | 100 | 48 |
| 4c | Cu(MeCN)4PF6 | Cs2CO3 | DCE | 100 | 0 |
| 5c | AgSbF6 | Cs2CO3 | DCE | 100 | 20 |
| 6 | [Rh(esp)]2 | LiOtBu | DCE | 100 | 53 |
| 7 | [Rh(esp)]2 | NaOtBu | DCE | 100 | 51 |
| 8 | [Rh(esp)]2 | K2CO3 | DCE | 100 | 62 |
| 9 | [Rh(esp)]2 | K3PO4 | DCE | 100 | 36 |
| 10 | [Rh(esp)]2 | Cs2CO3 | MeCN | 100 | 0 |
| 11 | [Rh(esp)]2 | Cs2CO3 | Dioxane | 100 | 75 |
| 12 | [Rh(esp)]2 | Cs2CO3 | THF | 100 | 69 |
| 13 | [Rh(esp)]2 | Cs2CO3 | DCE | 90 | 70 |
| 14 | [Rh(esp)]2 | Cs2CO3 | DCE | 110 | 80 |
| 15 | [Rh(esp)]2 | Cs2CO3 | DCE | 120 | 49 |
| 16d | [Rh(esp)]2 | Cs2CO3 | DCE | 100 | 78 |
| 17e | [Rh(esp)]2 | Cs2CO3 | DCE | 100 | 72 |
| 18f | [Rh(esp)]2 | Cs2CO3 | DCE | 100 | 80 |
| 19g | [Rh(esp)]2 | Cs2CO3 | DCE | 100 | 76 |
| Entry | Catalyst | Base | Solvent | Temperature/℃ | Yieldb/% |
|---|---|---|---|---|---|
| 1 | [Rh(OAc)2]2 | Cs2CO3 | DCE | 100 | 30 |
| 2 | [Rh(esp)]2 | Cs2CO3 | DCE | 100 | 81 |
| 3 | [Rh(OPiv)2]2 | Cs2CO3 | DCE | 100 | 48 |
| 4c | Cu(MeCN)4PF6 | Cs2CO3 | DCE | 100 | 0 |
| 5c | AgSbF6 | Cs2CO3 | DCE | 100 | 20 |
| 6 | [Rh(esp)]2 | LiOtBu | DCE | 100 | 53 |
| 7 | [Rh(esp)]2 | NaOtBu | DCE | 100 | 51 |
| 8 | [Rh(esp)]2 | K2CO3 | DCE | 100 | 62 |
| 9 | [Rh(esp)]2 | K3PO4 | DCE | 100 | 36 |
| 10 | [Rh(esp)]2 | Cs2CO3 | MeCN | 100 | 0 |
| 11 | [Rh(esp)]2 | Cs2CO3 | Dioxane | 100 | 75 |
| 12 | [Rh(esp)]2 | Cs2CO3 | THF | 100 | 69 |
| 13 | [Rh(esp)]2 | Cs2CO3 | DCE | 90 | 70 |
| 14 | [Rh(esp)]2 | Cs2CO3 | DCE | 110 | 80 |
| 15 | [Rh(esp)]2 | Cs2CO3 | DCE | 120 | 49 |
| 16d | [Rh(esp)]2 | Cs2CO3 | DCE | 100 | 78 |
| 17e | [Rh(esp)]2 | Cs2CO3 | DCE | 100 | 72 |
| 18f | [Rh(esp)]2 | Cs2CO3 | DCE | 100 | 80 |
| 19g | [Rh(esp)]2 | Cs2CO3 | DCE | 100 | 76 |

| [1] |
(a) Hagmann, W. K. J. Med. Chem. 2008, 51, 4359.
doi: 10.1021/jm800219f |
|
(b) Fujiwara, T.; O'Hagan, D. J. Fluorine Chem. 2014, 167, 16.
doi: 10.1016/j.jfluchem.2014.06.014 |
|
|
(c) Alonso, C.; Martínez de Marigorta, E.; Rubiales, G.; Palacios, F. Chem. Rev. 2015, 115, 1847.
doi: 10.1021/cr500368h |
|
|
(d) Meanwell, N. A. J. Med. Chem. 2018, 61, 5822.
doi: 10.1021/acs.jmedchem.7b01788 |
|
| [2] |
(a) Yang, Y.; You, Z.; Qing, F. Acta Chim. Sinica 2012, 70, 2323. (in Chinese)
doi: 10.6023/A12090668 pmid: 33237116 |
|
( 杨义, 游正伟, 卿凤翎, 化学学报 2012, 70, 2323.)
doi: 10.6023/A12090668 pmid: 33237116 |
|
|
(b) Carter, N. J.; Keating, G. Drugs 2007, 67, 2277.
doi: 10.2165/00003495-200767150-00010 pmid: 33237116 |
|
|
(c) Gagnadoux, F.; Pape, A. L.; Urban, T.; Montharu, J.; Vecellio, L.; Dubus, J. C.; Leblond, V.; Diot, P.; Grimbert, D.; Racineux, J. L.; Lemarié, E. J. Aerosol Med. 2005, 18, 198.
pmid: 33237116 |
|
|
(d) Barrett, S. D.; Holt, M. C.; Kramer, J. B.; Germain, B.; Ho, C. S.; Ciske, F. L.; Kornilov, A.; Colombo, J. M.; Uzieblo, A.; O’Malley, J. P.; Owen, T. A.; Stein, A. J.; Morano, M. I. J. Med. Chem. 2019, 62, 4731.
doi: 10.1021/acs.jmedchem.9b00336 pmid: 33237116 |
|
|
(e) Alter, C.; Hoge, B. J. Appl. Polym. Sci. 2018, 135, 46765.
doi: 10.1002/app.46765 pmid: 33237116 |
|
|
(f) Wang, X.; Lei, J.; Li, G.; Meng, J.; Li, C.; Li, J.; Sun, K. Org. Biomol. Chem. 2020, 18, 9762.
doi: 10.1039/d0ob02168g pmid: 33237116 |
|
|
(g) Wang, X.; Lei, J.; Liu, Y.; Ye, Y.; Li, J.; Sun, K. Org. Chem. Front. 2021, 8, 2079.
doi: 10.1039/D0QO01629B pmid: 33237116 |
|
|
(h) Zhao, F.; Guo, S.; Zhang, Y.; Sun, T.; Yang, B.; Ye, Y.; Sun, K. Org. Chem. Front. 2021, 8, 6895.
doi: 10.1039/D1QO01425K pmid: 33237116 |
|
|
(i) Sun, K.; Wang, S.; Feng, R.; Zhang, Y.; Wang, X.; Zhang, Z.; Zhang, B. Org. Lett. 2019, 21, 2052.
doi: 10.1021/acs.orglett.9b00240 pmid: 33237116 |
|
| [3] |
Hu, M.; Ni, C.; Hu, J. J. Am. Chem. Soc. 2012, 134, 15257.
doi: 10.1021/ja307058c |
| [4] |
(a) Hu, M.; He, Z.; Gao, B.; Li, L.; Ni, C.; Hu, J. J. Am. Chem. Soc. 2013, 135, 17302.
doi: 10.1021/ja409941r |
|
(b) Hu, M.; Ni, C.; Li, L.; Han, Y.; Hu, J. J. Am. Chem. Soc. 2015, 137, 14496.
doi: 10.1021/jacs.5b09888 |
|
|
(c) Hu, M.; Rong, J.; Miao, W.; Ni, C.; Han, Y.; Hu, J. Org. Lett. 2014, 16, 2030.
doi: 10.1021/ol500612n |
|
|
(d) Hu, M.; Xie, Q.; Li, X.; Ni, C.; Hu, J. Chin. J. Chem. 2016, 34, 469.
doi: 10.1002/cjoc.201600004 |
|
|
(e) Han, X.; Liu, X.; Bao, Y.; Song, H.; Zhao, Y.-R.; Wang, X.; Zhang, J.; Liu, L.; Duan, X.-H.; Hu, J.; Hu, M. Org. Chem. Front. 2022, 9, 204.
doi: 10.1039/D1QO01654G |
|
| [5] |
Yuan, W.; Eriksson, L.; Szabó, K. J. Angew. Chem., Int. Ed. 2016, 55, 8410.
doi: 10.1002/anie.201602137 |
| [6] |
Hu, X.-Q.; Han, J.-B.; Zhang, C.-P. Eur. J. Org. Chem. 2017, 2, 324.
|
| [7] |
Ma, Q.; Tsui, G. C. Org. Chem. Front. 2019, 6, 27.
doi: 10.1039/C8QO00834E |
| [8] |
(a) Yang, J.; Wang, J.; Huang, H.; Qin, G.; Jiang, Y.; Xiao, T. Org. Lett. 2019, 21, 2654.
doi: 10.1021/acs.orglett.9b00647 |
|
(b) Wang, J.; Yu, J.; Chen, J.; Jiang, Y.; Xiao, T. Org. Biomol. Chem. 2021, 19, 6974.
doi: 10.1039/D1OB01129D |
|
| [9] |
Murray, B. A. Reactions of Aldehydes and Ketones and their Derivatives. In Organic Reaction Mechanisms 2015, Ed.: Knipe, A. C., John Wiley & Sons Ltd., 2019, Chapter 1.
|
| [10] |
(a) Radolko, J.; Ehlers, P.; Langera, P. Adv. Synth. Catal. 2021, 363, 3616.
doi: 10.1002/adsc.202100332 |
|
(b) Lv, Y.; Meng, J.; Li, C.; Wang, X.; Ye, Y.; Sun, K. Adv. Synth. Catal. 2021, 363, 5235.
doi: 10.1002/adsc.202101184 |
|
|
(c) Xia, Y.; Wang, J. J. Am. Chem. Soc. 2020, 142, 10592.
doi: 10.1021/jacs.0c04445 |
|
|
(d) Wang, H.; Deng, Y.-H.; Shao, Z. Synthesis 2018, 50, 2281.
doi: 10.1055/s-0036-1591993 |
|
|
(e) Xia, Y.; Wang, J. Chem. Soc. Rev. 2017, 46, 2306.
doi: 10.1039/C6CS00737F |
|
|
(f) Xia, Y.; Qiu, D.; Wang, J. Chem. Rev. 2017, 117, 13810.
doi: 10.1021/acs.chemrev.7b00382 |
|
|
(g) Kai, X.; Chong, S.; Shang, S. Chin. J. Org. Chem. 2015, 35, 294. (in Chinese)
doi: 10.6023/cjoc201409034 |
|
|
( 许恺, 沈冲, 单尚, 有机化学 2015, 35, 294.)
doi: 10.6023/cjoc201409034 |
|
|
(h) Shao, Z.; Zhang, H. Chem. Soc. Rev. 2012, 41, 560.
doi: 10.1039/C1CS15127D |
|
| [11] |
(a) Yan, K.; He, H.; Li, J.; Luo, Y.; Lai, R.; Guo, L.; Wu, Y. Chin. Chem. Lett. 2021, 32, 3984.
doi: 10.1016/j.cclet.2021.05.031 pmid: 21268646 |
|
(b) Ning, Y.; Wang, H.; Sivaguru, P.; Li, S.; Zanoni, G. S.; Nolan, P.; Bi, X. Green Chem. 2021, 23, 7976.
doi: 10.1039/D1GC02749B pmid: 21268646 |
|
|
(c) Wang, H.-W.; Ning, Y.-Q.; Sun, Y.; Sivaguru, P.; Bi, X. Org. Lett. 2020, 22, 2012.
doi: 10.1021/acs.orglett.0c00395 pmid: 21268646 |
|
|
(d) Hossain, M.; Wang, K.; Ye, F.; Zhang, Y.; Wang, J. Chin. J. Catal. 2017, 38, 115.
doi: 10.1016/S1872-2067(16)62565-2 pmid: 21268646 |
|
|
(e) Zhou, L.; Shi, Y.; Xiao, Q.; Liu, Y.; Ye, F.; Zhang, Y.; Wang, J. Org. Lett. 2011, 13, 968.
doi: 10.1021/ol103009n pmid: 21268646 |
|
|
(f) Liu, Z.; Tan, H.; Wang, L.; Fu, T.; Xia, Y.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2015, 54, 3056.
doi: 10.1002/anie.201409982 pmid: 21268646 |
|
|
(g) Xiao, T.; Dong, X.; Zhou, L. Org. Biomol. Chem. 2013, 11, 1490.
doi: 10.1039/C2OB26867A pmid: 21268646 |
|
|
(h) Plaza, M.; Valdés, C. J. Am. Chem. Soc. 2016, 138, 12061.
doi: 10.1021/jacs.6b08116 pmid: 21268646 |
|
| [12] |
(a) Liu, Y.; Zhang, Z.; Zhang, S.; Zhang, Y.; Wang, J.; Zhang, Z. Chem. Asian J. 2018, 13, 3658.
doi: 10.1002/asia.201801324 pmid: 21341655 |
|
(b) Xiao, Q.; Ling, L.; Ye, F.; Tan, R.; Tian, L.; Zhang, Y.; Li, Y.; Wang, J. J. Org. Chem. 2013, 78, 3879.
doi: 10.1021/jo4002883 pmid: 21341655 |
|
|
(c) Zhao, X.; Wu, G.; Zhang, Y.; Wang, J. J. Am. Chem. Soc. 2011, 133, 3296.
doi: 10.1021/ja111249p pmid: 21341655 |
|
|
(d) Zeng, H.; Luo, Z.; Han, X.; Li, C.-J. Org. Lett. 2019, 21, 5948.
doi: 10.1021/acs.orglett.9b02072 pmid: 21341655 |
|
|
(e) Jha, A. K.; Jain, N. Chem. Commun. 2016, 52, 1831.
doi: 10.1039/C5CC07833D pmid: 21341655 |
|
|
(f) Teng, Q.; Hu, J.; Ling, L.; Sun, R.; Dong, J.; Chen, S.; Zhang, H. Org. Biomol. Chem. 2014, 12, 7721.
doi: 10.1039/C4OB01093K pmid: 21341655 |
|
|
(g) Yao, T.; Hirano, K.; Satoh, T.; Miura, M. Angew. Chem., Int. Ed. 2012, 51, 775.
doi: 10.1002/anie.201106825 pmid: 21341655 |
|
|
(h) Barluenga, J.; Tomás-Gamasa, M.; Aznar, F.; Valdés, C. Angew. Chem., Int. Ed. 2010, 49, 4993.
doi: 10.1002/anie.201001704 pmid: 21341655 |
|
|
(i) Ding, Q.; Cao, B.; Yuan, J.; Liu, X.; Peng, Y. Org. Biomol. Chem. 2011, 9, 748.
doi: 10.1039/C0OB00639D pmid: 21341655 |
|
|
(j) Liu, Z.; Tan, H.; Fu, T.; Xia, Y.; Qiu, D.; Zhang, Y.; Wang, J. J. Am. Chem. Soc. 2015, 137, 12800.
doi: 10.1021/jacs.5b09135 pmid: 21341655 |
|
|
(k) Huo, J.; Zhong, K.; Xue, Y.; Lyu, M.; Ping, Y.; Liu, Z.; Lan, Y.; Wang, J. J. Am. Chem. Soc. 2021, 143, 12968.
doi: 10.1021/jacs.1c05879 pmid: 21341655 |
|
| [13] |
(a) Barluenga, J.; Moriel, P.; Valdés, C.; Aznar, F. Angew. Chem., Int. Ed. 2007, 46, 5587.
doi: 10.1002/anie.200701815 pmid: 23788377 |
|
(b) Barluenga, J.; Escribano, M.; Aznar, F.; Valdés, C. Angew. Chem., Int. Ed. 2010, 49, 6856.
doi: 10.1002/anie.201003450 pmid: 23788377 |
|
|
(c) Florentino, L.; Aznar, F.; Valdés, C. Chem.-Eur. J. 2013, 19, 10506.
doi: 10.1002/chem.201301057 pmid: 23788377 |
|
|
(d) Zhao, X.; Jing, J.; Lu, K.; Zhang, Y.; Wang, J. Chem. Commun. 2010, 46, 1724.
doi: 10.1039/b925590g pmid: 23788377 |
|
|
(e) Hu, F.; Xia, Y.; Ye, F.; Liu, Z.; Ma, C.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2014, 53, 1364.
doi: 10.1002/anie.201309650 pmid: 23788377 |
|
|
(f) Zhou, L.; Ye, F.; Ma, J.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2011, 50, 3510.
doi: 10.1002/anie.201007224 pmid: 23788377 |
|
|
(g) Xiao, Q.; Xia, Y.; Li, H.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2011, 50, 1114.
doi: 10.1002/anie.201005741 pmid: 23788377 |
|
|
(h) Ye, F. ; Hossain, M. L. ; Xu, Y. ; Ma, X. ; Xiao, Q. ; Zhang, Y. Wang, J. Chem. Asian J. 2013, 8, 1404.
doi: 10.1002/asia.201300340 pmid: 23788377 |
|
|
(i) Jiang, H.; He, L.; Li, X.; Chen, H.; Wu, W.; Fu, W. Chem. Commun. 2013, 49, 9218.
doi: 10.1039/c3cc43593h pmid: 23788377 |
|
|
(j) Wang, K.; Chen, S.; Zhang, H.; Xu, S.; Ye, F.; Zhang, Y.; Wang, J. Org. Biomol. Chem. 2016, 14, 3809.
doi: 10.1039/C6OB00454G pmid: 23788377 |
|
|
(k) Chen, Z.-S.; Duan, X.-H.; Wu, L.-Y.; Ali, S.; Ji, K.-G.; Zhou, P.-X.; Liu, X.-Y.; Liang, Y.-M. Chem. Eur. J. 2011, 17, 6918.
doi: 10.1002/chem.201100248 pmid: 23788377 |
|
|
(l) Chen, H.; Huang, L.; Fu, W.; Liu, X.; Jiang, H. Chem. Eur. J. 2012, 18, 10497.
doi: 10.1002/chem.201200949 pmid: 23788377 |
|
|
(m) Zhang, Z.; Yu, W.; Zhou, Q.; Li, T.; Zhang, Y.; Wang, J. Chin. J. Chem. 2016, 34, 473.
doi: 10.1002/cjoc.201500889 pmid: 23788377 |
|
|
(n) Zhang, Z.; Zhou, Q.; Yu, W.; Li, T.; Wu, G.; Zhang, Y.; Wang, J. Org. Lett. 2015, 17, 2474.
doi: 10.1021/acs.orglett.5b00980 pmid: 23788377 |
|
|
(o) Mao, M.; Zhang, L.; Chen, Y.-Z.; Zhu, J.; Wu, L. ACS Catal. 2017, 7, 181.
doi: 10.1021/acscatal.6b02972 pmid: 23788377 |
|
|
(p) Ping, Y.; Wang, R.; Wang, Q.; Chang, T.; Huo, J.; Lei, M.; Wang, J. J. Am. Chem. Soc. 2021, 143, 9769.
doi: 10.1021/jacs.1c02331 pmid: 23788377 |
|
| [14] |
(a) Xiao, T.; Mei, M.; He, Y.; Zhou, L. Chem. Commun. 2018, 54, 8865.
doi: 10.1039/C8CC04609C |
|
(b) Xiao, T.; Li, L.; Xie, Y.; Mao, Z.-W.; Zhou, L. Org. Lett. 2016, 18, 1004.
doi: 10.1021/acs.orglett.6b00119 |
|
|
(c) Xiao, T.; Li, L.; Zhou, L. J. Org. Chem. 2016, 81, 7908.
doi: 10.1021/acs.joc.6b01620 |
|
|
(d) Huang, H.; Chen, J.; Jiang, Y.; Xiao, T. Org. Chem. Front. 2021, 8, 5955.
doi: 10.1039/D1QO01024G |
| [1] | 文思, 丁宇浩, 田青于, 葛进, 程国林. 铑(III)催化苯甲亚胺酸乙酯和CF3-亚胺氧锍叶立德C—H 活化/环化反应合成CF3-1H-苯并[de][1,8]萘吡啶[J]. 有机化学, 2024, 44(1): 291-300. |
| [2] | 张彦波, 孙萌. 铑催化碳酸亚乙烯酯与吲哚啉C(7)位C—H甲酰甲基化反应[J]. 有机化学, 2023, 43(8): 2905-2912. |
| [3] | 汤振, 皮超, 吴养洁, 崔秀灵. 铑催化2-芳基-2H-吲唑与硫叶立德的酰甲基化/串联环化反应高效构建6-芳基吲唑并[2,3-a]喹啉类衍生物[J]. 有机化学, 2023, 43(3): 1187-1196. |
| [4] | 刘晓洁, 徐必平, 苏伟平. 铑催化羧酸原位生成酰氟的脱羰Suzuki-Miyaura偶联[J]. 有机化学, 2022, 42(7): 2184-2191. |
| [5] | 戴雨倩, 李兴伟, 刘丙贤. 三价铑催化通过环己二酮高效构建异香豆素类化合物[J]. 有机化学, 2021, 41(11): 4476-4483. |
| [6] | 赵森, 李淳朴, 许斌, 柳红. 铑催化碳氢二氟烯丙基化/N-碘代丁二酰亚胺介导的环化反应构建含氟3,4-二氢嘧啶并[1,6-a]吲哚-1(2H)-酮衍生物[J]. 有机化学, 2020, 40(6): 1549-1562. |
| [7] | 王志惠, 张振锋, 刘燕刚, 张万斌. 烯醇酯的不对称催化氢化研究进展[J]. 有机化学, 2016, 36(3): 447-459. |
| [8] | 王英杰, 张振锋, 张万斌. 环状脱氢氨基酸及其衍生物的不对称催化氢化研究[J]. 有机化学, 2015, 35(3): 528-538. |
| [9] | 王亮, 瞿星, 李站, 彭望明. 铑(III)催化吲哚甲酰胺衍生物与末端炔烃的C—H活化/环化反应[J]. 有机化学, 2015, 35(3): 688-697. |
| [10] | 罗人仕,杨定乔. 铑催化环加成反应的研究进展[J]. 有机化学, 2007, 27(08): 958-969. |
| [11] | 王欢,杨定乔,莫海洪. 铑催化不对称1,4-共轭加成反应的研究进展[J]. 有机化学, 2007, 27(07): 806-818. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||