有机化学 ›› 2022, Vol. 42 ›› Issue (10): 3390-3397.DOI: 10.6023/cjoc202206053 上一篇 下一篇
所属专题: 不对称催化专辑
研究论文
李芳洁a,b, 卢斌b, 刘阳a,*( ), 王晓明b,c,*(
), 王晓明b,c,*( )
)
收稿日期:2022-06-28
修回日期:2022-08-26
发布日期:2022-11-02
通讯作者:
刘阳, 王晓明
基金资助:
Fangjie Lia,b, Bin Lub, Yang Liua( ), Xiaoming Wangb,c(
), Xiaoming Wangb,c( )
)
Received:2022-06-28
Revised:2022-08-26
Published:2022-11-02
Contact:
Yang Liu, Xiaoming Wang
Supported by:文章分享
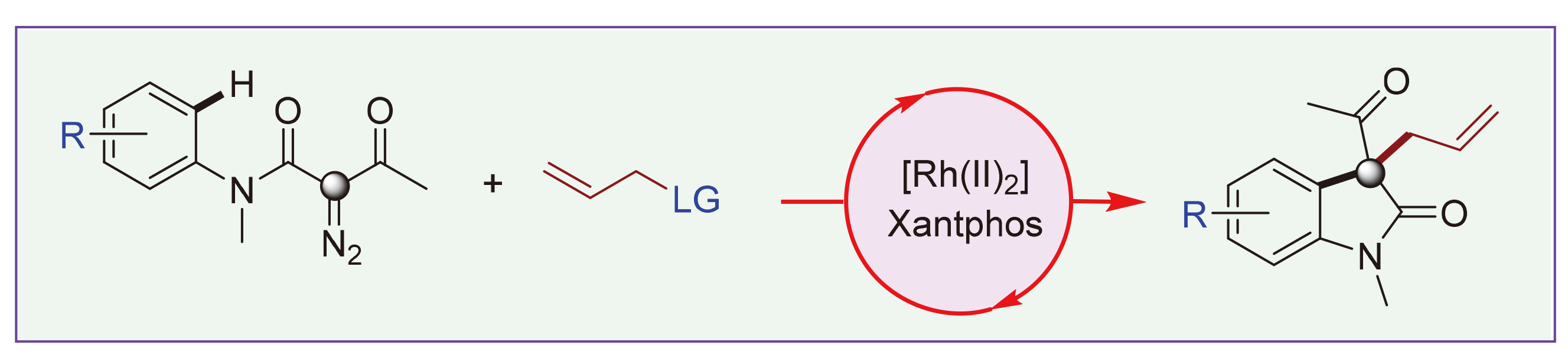
从易于获得的N-芳基-α-重氮-β-酮酰胺和烯丙基化合物出发, 利用新型的[Rh2]/Xantphos催化体系一锅法合成了各种3-酰基-3-烯丙基的氧化吲哚衍生物. 这项工作的优点包括操作简便、反应条件温和、官能团兼容性良好和产物易于衍生化等. 初步的机理研究表明, 该反应可能经历卡宾诱导的C—H官能团化和Rh(II)/Xantphos催化的烯丙基烷基化串联过程. 此外, 选择Xantphos作为配体对于实现体系中的烯丙基烷基化过程至关重要.
李芳洁, 卢斌, 刘阳, 王晓明. 双铑(II)/Xantphos催化的C—H官能团化/烯丙基烷基化串联: 从N-芳基-α-重氮-β-酮酯简便制备3-酰基-3-烯丙基氧化吲哚衍生物[J]. 有机化学, 2022, 42(10): 3390-3397.
Fangjie Li, Bin Lu, Yang Liu, Xiaoming Wang. Dirhodium/Xantphos-Catalyzed Tandem C—H Functionalization/Allylic Alkylation: Direct Access to 3-Acyl-3-allyl Oxindole Derivatives from N-Aryl-α-diazo-β-keto Amides[J]. Chinese Journal of Organic Chemistry, 2022, 42(10): 3390-3397.
| Entry | [Rh] cat. | Ligand | Base | Solvent | Yieldb/% | |
|---|---|---|---|---|---|---|
| 3a | 4a | |||||
| 1 | Rh2(Oct)4 | Xantphos | Cs2CO3 | DCE | — | 51 |
| 2 | Rh2(Oct)4 | Xantphos | — | DCE | 83 | 0 |
| 3 | Rh2(Oct)4 | Xantphos | KOAc | DCE | — | 74 |
| 4 | Rh2(Oct)4 | Xantphos | KOAc | PhCF3 | — | 88 |
| 5 | Rh2(OAc)4 | Xantphos | KOAc | PhCF3 | — | <1 |
| 6 | Rh2(TFA)4 | Xantphos | KOAc | PhCF3 | — | 7 |
| 7 | Rh(COD)2BF4 | Xantphos | KOAc | PhCF3 | N.R.c | N.R.c |
| 8 | Rh2(Oct)4 | BINAP | KOAc | PhCF3 | — | 9 |
| 9 | Rh2(Oct)4 | dppb | KOAc | PhCF3 | — | 7 |
| 10 | Rh2(Oct)4 | PPh3 | KOAc | PhCF3 | — | <5 |
| 11 | Rh2(Oct)4 | iPr-NHCd | KOAc | PhCF3 | — | <5 |
| 12e | Rh2(Oct)4 | Xantphos | KOAc | PhCF3 | — | 83 |
| 13f | Rh2(Oct)4 | Xantphos | KOAc | PhCF3 | — | 84 |
| 14 | — | Xantphos | KOAc | PhCF3 | — | N.R. |
| 15 | Rh2(Oct)4 | — | KOAc | PhCF3 | — | N.R. |
| Entry | [Rh] cat. | Ligand | Base | Solvent | Yieldb/% | |
|---|---|---|---|---|---|---|
| 3a | 4a | |||||
| 1 | Rh2(Oct)4 | Xantphos | Cs2CO3 | DCE | — | 51 |
| 2 | Rh2(Oct)4 | Xantphos | — | DCE | 83 | 0 |
| 3 | Rh2(Oct)4 | Xantphos | KOAc | DCE | — | 74 |
| 4 | Rh2(Oct)4 | Xantphos | KOAc | PhCF3 | — | 88 |
| 5 | Rh2(OAc)4 | Xantphos | KOAc | PhCF3 | — | <1 |
| 6 | Rh2(TFA)4 | Xantphos | KOAc | PhCF3 | — | 7 |
| 7 | Rh(COD)2BF4 | Xantphos | KOAc | PhCF3 | N.R.c | N.R.c |
| 8 | Rh2(Oct)4 | BINAP | KOAc | PhCF3 | — | 9 |
| 9 | Rh2(Oct)4 | dppb | KOAc | PhCF3 | — | 7 |
| 10 | Rh2(Oct)4 | PPh3 | KOAc | PhCF3 | — | <5 |
| 11 | Rh2(Oct)4 | iPr-NHCd | KOAc | PhCF3 | — | <5 |
| 12e | Rh2(Oct)4 | Xantphos | KOAc | PhCF3 | — | 83 |
| 13f | Rh2(Oct)4 | Xantphos | KOAc | PhCF3 | — | 84 |
| 14 | — | Xantphos | KOAc | PhCF3 | — | N.R. |
| 15 | Rh2(Oct)4 | — | KOAc | PhCF3 | — | N.R. |
| [1] |
(a) Galliford, C. V.; Scheidt, K. A. Angew. Chem., Int. Ed. 2007, 46, 8748.
doi: 10.1002/anie.200701342 |
|
(b) Yu, B.; Yu, D.-Q.; Liu, H.-M. Eur. J. Med. Chem. 2015, 97, 673.
doi: 10.1016/j.ejmech.2014.06.056 |
|
| [2] |
(a) Zhou, F.; Liu, Y.-L.; Zhou, J. Adv. Synth. Catal. 2010, 352, 1381.
doi: 10.1002/adsc.201000161 pmid: 22899437 |
|
(b) Dalpozzo, R.; Bartoli, G.; Bencivenni, G. Chem. Soc. Rev. 2012, 41, 7247.
doi: 10.1039/c2cs35100e pmid: 22899437 |
|
|
(c) Liu, Y.-L.; Wang, X.; Zhao, Y.-L.; Zhu, F.; Zeng, X.-P.; Chen, L.; Wang, C.-H.; Zhao, X.-L.; Zhou, J. Angew. Chem., Int. Ed. 2013, 52, 13735.
doi: 10.1002/anie.201307250 pmid: 22899437 |
|
|
(d) Cao, Z.-Y.; Wang, X.; Tan, C.; Zhao, X.-L.; Zhou, J.; Ding, K. J. Am. Chem. Soc. 2013, 135, 8197.
doi: 10.1021/ja4040895 pmid: 22899437 |
|
|
(e) Zhou, F.; Tan, C.; Tang, J.; Zhang, Y.-Y.; Gao, W.-M.; Wu, H.-H.; Yu, Y.-H.; Zhou, J. J. Am. Chem. Soc. 2013, 135, 10994.
doi: 10.1021/ja4066656 pmid: 22899437 |
|
|
(f) Zeng, X.-P.; Cao, Z-Y.; Wang, Y.-H.; Zhou, F.; Zhou, J. Chem. Rev. 2016, 116, 7330.
doi: 10.1021/acs.chemrev.6b00094 pmid: 22899437 |
|
|
(g) Cao, Z.-Y.; Zhou, F.; Zhou, J. Acc. Chem. Res. 2018, 51, 1443.
doi: 10.1021/acs.accounts.8b00097 pmid: 22899437 |
|
|
(h) Xu, P.-W.; Yu, J.-S.; Chen, C.; Cao, Z.-Y.; Zhou, F.; Zhou, J. ACS Catal. 2019, 9, 1820.
doi: 10.1021/acscatal.8b03694 pmid: 22899437 |
|
|
(i) Marchese, A. D.; Larin, E. M.; Mirabi, B.; Lautens, M. Acc. Chem. Res. 2020, 53, 1605.
doi: 10.1021/acs.accounts.0c00297 pmid: 22899437 |
|
|
(j) Liu, T. T.; Zhang, X. Y.; Miao, Z. W. Chin. J. Org. Chem. 2021, 41, 3965. (in Chinese).
doi: 10.6023/cjoc202104008 pmid: 22899437 |
|
|
(刘奕彤, 张茜苑, 苗志伟, 有机化学, 2021, 41, 3965.)
pmid: 22899437 |
|
| [3] |
(a) Trost, B. M.; Van Vranken, D. L. Chem. Rev. 1996, 96, 395.
doi: 10.1021/cr9409804 pmid: 32756671 |
|
(b) Cheng, Q.; Tu, H.-F.; Zheng, C.; Qu, J.-P.; Helmchen, G.; You, S.-L. Chem. Rev. 2019, 119, 1855.
doi: 10.1021/acs.chemrev.8b00506 pmid: 32756671 |
|
|
(c) Butt, N. A.; Zhang, W. Chem. Soc. Rev. 2015, 44, 7929.
doi: 10.1039/C5CS00144G pmid: 32756671 |
|
|
(d) Turnbull, B. W. H.; Evans, P. A. J. Org. Chem. 2018, 83, 11463.
doi: 10.1021/acs.joc.8b00583 pmid: 32756671 |
|
|
(e) Zhang, H.; Gu, Q.; You, S.-L. Chin. J. Org. Chem. 2019, 39, 15. (in Chinese).
pmid: 32756671 |
|
|
(张慧君, 顾庆, 游书力, 有机化学, 2019, 39, 15.)
doi: 10.6023/cjoc201809037 pmid: 32756671 |
|
|
(f) Huang, H.-M.; Bellotti, P.; Glorius, F. Chem. Soc. Rev. 2020, 49, 6186.
doi: 10.1039/d0cs00262c pmid: 32756671 |
|
| [4] |
(a) Wei, F.; Song, C.-L.; Ma, Y.-D.; Zhou, L.; Tung, C.-H.; Xu, Z.-H. Sci. Bull. 2015, 60, 1479.
doi: 10.1007/s11434-015-0874-0 pmid: 26658761 |
|
(b) Liu,
doi: 10.1039/c5cs00821b pmid: 26658761 |
|
| [5] |
For selected examples, see: a , Qiu, H.; Li, M.; Jiang, L.-Q.; Lv, F.-P.; Zan, L.; Zhai, C.-W.; Doyle, M. P.; Hu, W.-H. Nat. Chem. 2012, 4, 733.
doi: 10.1038/nchem.1406 pmid: 35756859 |
|
(b) Xi, Y.; Su, Y.; Yu, Z.; Dong, B.; McClain, E. J.; Lan, Y.; Shi, X. Angew. Chem., Int. Ed. 2014, 53, 9817.
doi: 10.1002/anie.201404946 pmid: 35756859 |
|
|
(c) Yu, Z.; Ma, B.; Chen, M.; Wu, H.-H.; Liu, L.; Zhang, J. J. Am. Chem. Soc. 2014, 136, 6904.
doi: 10.1021/ja503163k pmid: 35756859 |
|
|
(d) Chen, D.-F.; Zhao, F.; Hu, Y.; Gong, L.-Z. Angew. Chem., Int. Ed. 2014, 53, 10763.
doi: 10.1002/anie.201406098 pmid: 35756859 |
|
|
(e) Chen, D.-F.; Zhang, C.-l.; Hu, Y.; Han, Z.-Y.; Gong, L.-Z. Org. Chem. Front. 2015, 2, 956.
doi: 10.1039/C5QO00151J pmid: 35756859 |
|
|
(f) Jing C.; Xing, D.; Hu, W. Org. Lett. 2015, 17, 4336.
doi: 10.1021/acs.orglett.5b02160 pmid: 35756859 |
|
|
(g) Xu, B.; Li, ML.; Zuo, X.-D.; Zhu, S.-F.; Zhou, Q.-L. J. Am. Chem. Soc. 2015, 137, 8700.
doi: 10.1021/jacs.5b05086 pmid: 35756859 |
|
|
(h) Yu, Z.; Li, Y.; Shi, J.; Ma, B.; Liu, L.; Zhang, J. Angew. Chem.,Int. Ed. 2016, 55, 14807.
doi: 10.1002/anie.201608937 pmid: 35756859 |
|
|
(i) Fructos, M. R.; Díaz-Requejo M. M.; Pérez P. J. Chem. Commun. 2016, 52, 7326.
doi: 10.1039/C6CC01958G pmid: 35756859 |
|
|
(j) Ma, B.; Chu, Z.; Huang, B.; Liu, Z.; Liu, L.; Zhang, J. Angew. Chem., Int. Ed. 2017, 56, 2749.
doi: 10.1002/anie.201611809 pmid: 35756859 |
|
|
(k) Fructos, M. R.; Besora, M.; Braga, A. A. C.; Díaz-Requejo, M. M.; Maseras, F.; Perez, P. J. Organometallics 2017, 36, 172.
doi: 10.1021/acs.organomet.6b00604 pmid: 35756859 |
|
|
(l) Kang, Z.; Zhang, D.; Xu, X.; Hu, W. Org. Lett. 2019, 21, 9878.
doi: 10.1021/acs.orglett.9b03787 pmid: 35756859 |
|
|
(m) Yu, Z.; Li, Y.; Zhang, P.; Liu, L.; Zhang, J. Chem. Sci. 2019, 10, 6553.
doi: 10.1039/C9SC01657K pmid: 35756859 |
|
|
(n) Jana, S.; Empel, C.; Pei, C.; Aseeva, P.; Nguyen, T. V.; Koenigs, R. M. ACS Catal. 2020, 10, 9925.
doi: 10.1021/acscatal.0c02230 pmid: 35756859 |
|
|
(o) Zhang, C.; Hong, K.; Pei, C.; Zhou, S.; Hu, W.; Hashmi, A. S. K.; Xu, X. Nat. Commun. 2021, 12, 1182.
doi: 10.1038/s41467-021-21335-9 pmid: 35756859 |
|
|
(p) Zhu, D.-X.; Xia, H.; Liu, J.-G.; Chung, L.-W.; Xu, M.-H. J. Am. Chem. Soc. 2021, 143, 2608.
doi: 10.1021/jacs.0c13191 pmid: 35756859 |
|
|
(q) Li, Z.; Chen, Y.; Wang, C.; Xu, G.; Shao, Y.; Zhang, X.; Sun, J. Angew. Chem., Int. Ed. 2021, 60, 25714.
doi: 10.1002/anie.202110430 pmid: 35756859 |
|
|
(r) Pizarro, J. D.; Schmidtke, I. L.; Nova, A.; Fructos, M. R.; Pérez, P. J. ACS Catal. 2022, 12, 6851.
doi: 10.1021/acscatal.2c01713 pmid: 35756859 |
|
|
(s) Jia, S.; Xing, D.; Zhang, D.; Hu, W. Angew. Chem., Int. Ed. 2014, 53, 13098.
doi: 10.1002/anie.201406492 pmid: 35756859 |
|
| [6] |
(a) Jing, C.; Xing, D.; Hu, W. Org. Lett. 2015, 17, 4336.
doi: 10.1021/acs.orglett.5b02160 |
|
(b) Kang, Z.; Zhang, D.; Xu, X.; Hu, W. Org. Lett. 2019, 21, 9878.
doi: 10.1021/acs.orglett.9b03787 |
|
| [7] |
(a) Yamamoto, K.; Qureshi, Z.; Tsoung, J.; Pisella, G.; Lautens, M. Org. Lett. 2016, 18, 4954.
pmid: 27632781 |
|
(b) Chen, L. H.; Ma, Y. T.; Yang, F.; Huang, X. Y.; Chen, S. W.; Ji, K.; Chen, Z. S. Adv. Synth. Cat. 2019, 361, 1307.
doi: 10.1002/adsc.201801346 pmid: 27632781 |
|
|
(c) Lee, Y. L.; Lee, K. R.; Xuan, Z.; Lee, S.-G. Bull. Korean Chem. Soc. 2021, 42, 537.
doi: 10.1002/bkcs.12211 pmid: 27632781 |
|
| [8] |
(a) Tsuji, J.; Minami, I.; Shimizu, I. Tetrahedron Lett. 1984, 25, 5157.
doi: 10.1016/S0040-4039(01)81551-3 pmid: 30183287 |
|
(b) Turnbull, B. W. H.; Evans, P. A. J. Org. Chem. 2018, 83, 11463.
doi: 10.1021/acs.joc.8b00583 pmid: 30183287 |
|
|
(c) Thoke, M. B.; Kang, Q. Synthesis 2019, 51, 2585.
doi: 10.1055/s-0037-1611784 pmid: 30183287 |
|
| [9] |
(a) Cheng, M.; Huang, X.-Y.; Yang, F.; Zhao, D.-M.; Ji, K.; Chen, Z.-S. Org. Lett. 2022, 24, 1237.
doi: 10.1021/acs.orglett.2c00073 |
|
For selected examples of RhII/Pd(0)dual catalyzed two-com- ponent reactions of diazo compounds with allylic compounds, see: (b) Chen, Z.-S.; Huang, X.-Y.; Gao, J.-M.; Ji, K. Org. Lett. 2016, 18, 5876.
doi: 10.1021/acs.orglett.6b02958 |
|
|
(c) Chen, Z.-S.; Huang, X.-Y.; Chen, L.-H.; Gao, J.-M.; Ji, K. ACS Catal. 2017, 7, 7902.
doi: 10.1021/acscatal.7b02909 |
|
|
(d) Huang, L.-Z.; Xuan, Z.; Jeon, H. J.; Du, Z.-T.; Kim, J. H.; Lee, S.-G. ACS Catal. 2018, 8, 7340.
doi: 10.1021/acscatal.8b01687 |
|
|
(e) Wang, X. X.; Huang, X. Y.; Lei, S. H.; Yang, F.; Gao, J. M.; Ji, K.; Chen, Z. S. Chem. Commun. 2020, 56, 782.
doi: 10.1039/C9CC08559A |
|
|
For an elegant RhII/Pd(0)/chiral phosphoric acid catalyzed three- component allylation of α-diazo carbonyl compounds with alcohols and allyl carbonates, see: (f) Kang, Z.; Chang, W.; Tian, X.; Fu, X.; Zhao, W.; Xu, X.; Liang, Y.; Hu, W. J. Am. Chem. Soc. 2021, 143, 20818.
doi: 10.1021/jacs.1c09148 |
|
| [10] |
(a) Lu, B.; Liang, X.; Zhang, J.; Wang, Z.; Peng, Q.; Wang, X. J. Am. Chem. Soc. 2021, 143, 11799.
doi: 10.1021/jacs.1c05701 |
|
(b) Yang, Y.; Lu, B.; Xu, G.; Wang, X. ACS Cent. Sci. 2022. 8, 581
doi: 10.1021/acscentsci.2c00004 |
|
| [11] |
For excellent reviews about the axial modification of dirhodium in catalysis, see: (a) Trindade, A. F.; Coelho, J. A. S.; Afonso, C. A. M.; Veiros, L. F.; Gois, P. M. P. ACS Catal. 2012, 2, 370.
doi: 10.1021/cs200597a |
|
(b) Hong, B.; Shi, L.; Li, L.; Zhan, S.; Gu, Z. Green Synth. Catal. 2022. doi.org/10.1016/j.gresc.2022.03.001.
|
|
| [12] |
For selected examples, see: (a) Wang, D.; Zhao, Y.; Yuan, C.; Wen, J.; Zhao, Y.; Shi, Z. Angew. Chem., Int. Ed. 2019, 58, 12529.
doi: 10.1002/anie.201906975 |
|
(b) Fu, L.; Li, S.; Cai, Z.; Ding, Y.; Guo, X.-Q.; Zhou, L.-P.; Yuan, D.; Sun, Q.-F.; Li, G. Nat. Catal. 2018, 1, 469.
doi: 10.1038/s41929-018-0080-y |
|
|
(c) Vora, H. U.; Silvestri, A. P.; Engelin, C. J.; Yu, J. Q. Angew. Chem., Int. Ed. 2014, 53, 2683.
|
|
|
(d) Kwak, J.; Kim, M.; Chang, S. J. Am. Chem. Soc. 2011, 133, 3780.
doi: 10.1021/ja111670s |
|
|
(e) Trindade, A. F.; Gois, P. M. P.; Veiros, L. F.; André, V.; Duarte, M. T.; Afonso, C. A. M.; Caddick, S.; Cloke, F. G. N. J. Org. Chem. 2008, 73, 4076.
doi: 10.1021/jo800087n |
|
|
(f) Gois, P. M. P.; Trindade, A. F.; Veiros, L. F.; André, V.; Duarte, M. T.; Afonso, C. A. M.; Caddick, S.; Cloke, F. G. N. Angew. Chem., Int. Ed. 2007, 46, 5750.
doi: 10.1002/anie.200700924 |
|
| [13] |
Moreno-Cabrerizo, C.; Ortega-Martínez, A.; Esteruelas, M. A.; López, A. M.; Nájera, C.; Sansano, J. M. Eur. J. Org. Chem. 2020, 2020, 3101
doi: 10.1002/ejoc.202000375 |
| [14] |
Mandal, T.; Chakraborti, G.; Karmakar, S.; Dash, J. Org. Lett. 2018, 20, 4759.
doi: 10.1021/acs.orglett.8b01827 |
| [15] |
(a) Guo, X.; Hu, W. Acc. Chem. Res. 2013, 46, 2427.
doi: 10.1021/ar300340k |
|
(b) Zhang, D.; Hu, W. Chem. Rec. 2017, 17, 739.
doi: 10.1002/tcr.201600124 |
| [1] | 岁丹丹, 岑南楠, 龚若蕖, 陈阳, 陈文博. 无支持电解质条件下连续流电化学合成三氟甲基化氧化吲哚[J]. 有机化学, 2023, 43(9): 3239-3245. |
| [2] | 许晓萍, 张翼飞, 莫小渝, 江俊. 铑催化3-重氮吲哚-2-亚胺与吡唑啉酮的C—H官能团化反应制备3-吡唑基吲哚[J]. 有机化学, 2023, 43(7): 2519-2527. |
| [3] | 鲍志成, 李慕尧, 王剑波. 铜催化芳基重氮乙酸酯与双[(频哪醇)硼基]甲烷的偶联反应[J]. 有机化学, 2023, 43(5): 1808-1814. |
| [4] | 潘振涛, 刘彤, 马永敏, 颜剑波, 王亚军. 布朗斯特酸/可见光氧化还原接力催化构建喹唑啉(硫)酮[J]. 有机化学, 2022, 42(9): 2823-2831. |
| [5] | 李心灵, 孟卫东, 徐修华, 黄焰根. 可见光诱导活化烯烃的芳基氟烷基化反应[J]. 有机化学, 2022, 42(6): 1820-1830. |
| [6] | 左鸿华, 钟芳锐. 亚稳醌类分子的活性调控与仿生催化反应[J]. 有机化学, 2022, 42(3): 665-678. |
| [7] | 任新意, 王广柱, 纪晓雷, 董开武. 一锅法合成两种含有季碳中心的氰基化合物[J]. 有机化学, 2022, 42(2): 526-533. |
| [8] | 谢阳, 宣俊. 重氮化合物作为自由基前体参与的光催化反应[J]. 有机化学, 2022, 42(12): 4247-4256. |
| [9] | 李森, 周磊. 可见光促进重氮化合物参与的自由基反应[J]. 有机化学, 2022, 42(12): 3944-3958. |
| [10] | 商铭洲, 张兰兰, 陈淼淼, 胡汪成, 何心伟, 陆红健. 铑催化的邻苯二甲酸酐与环状2-重氮-1,3-二酮和甲醇的串联C—H活化/跨环偶联/环化反应合成酯基官能化的并环异香豆素类化合物的研究[J]. 有机化学, 2022, 42(11): 3816-3823. |
| [11] | 张骁勇, 于丽丽, 高俊芳, 宫岳, 赵玉龙. 1,8-二氮杂双环[5.4.0]十一碳-7-烯(DBU)驱动的双分子硝基烷烃对重氮化合物的亲核加成反应: 多官能化腙和四氢哒嗪化合物的合成[J]. 有机化学, 2022, 42(11): 3704-3713. |
| [12] | 滕明瑜, 韩涛, 黄恩和, 叶龙武. 金属卡宾参与的对映选择性去对称化反应研究进展[J]. 有机化学, 2022, 42(10): 3295-3301. |
| [13] | 王东琳, 阚玲珑, 马玉道, 刘磊. 叔丁醇钠催化的δ-腈基-δ-芳基-双取代的对亚甲基苯醌和二芳基氧磷的磷氢化反应研究[J]. 有机化学, 2021, 41(8): 3192-3203. |
| [14] | 蔡宝贵, 宣俊. 可见光诱导重氮化合物产生卡宾及其官能化反应[J]. 有机化学, 2021, 41(12): 4565-4574. |
| [15] | 郭欣, 郭亚军, 孔德志, 卢会杰, 华远照, 王敏灿. 四氢呋喃螺氧化吲哚衍生物的一锅法高效合成[J]. 有机化学, 2020, 40(7): 1999-2007. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||