有机化学 ›› 2022, Vol. 42 ›› Issue (12): 4247-4256.DOI: 10.6023/cjoc202207016 上一篇 下一篇
所属专题: 自由基化学专辑
综述与进展
收稿日期:2022-07-07
修回日期:2022-08-09
发布日期:2022-08-17
通讯作者:
宣俊
基金资助:Received:2022-07-07
Revised:2022-08-09
Published:2022-08-17
Contact:
Jun Xuan
Supported by:文章分享

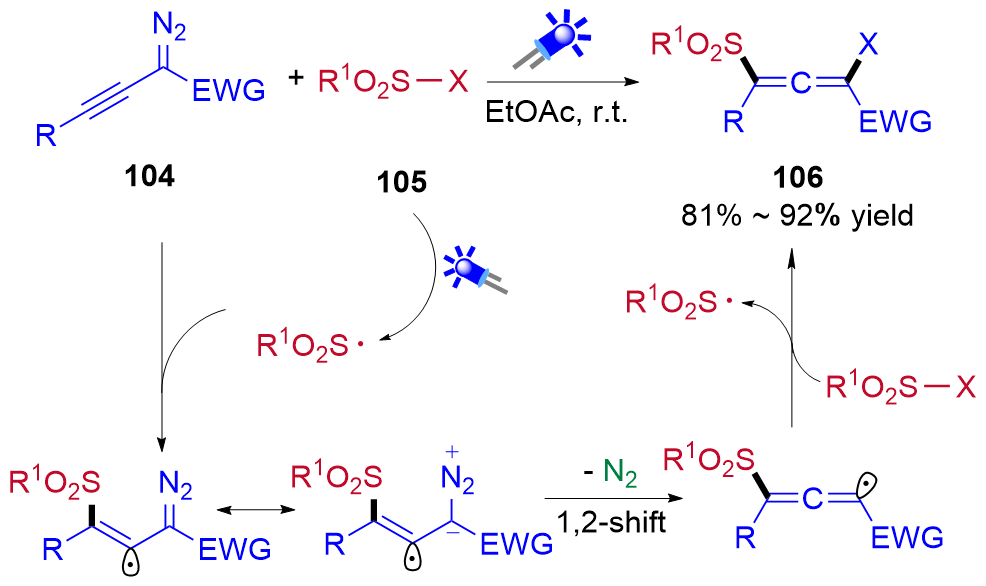
重氮化合物作为一种用途广泛的合成子而备受化学家的青睐. 在有机合成中, 重氮化合物可以作为卡宾前体1,3-偶极子、C-亲核试剂、末端N-亲电试剂以及自由基中间体等. 近年来, 可见光催化重氮化合物产生自由基及其后续官能化反应取得了长足的进展. 重点综述了近年来在可见光催化下, 重氮化合物作为不同自由基前体参与的有机合成反应. 主要包括光照产生重氮甲基自由基、碳自由基、卡拜自由基和联烯自由基等. 最后对该领域未来的发展方向和面临的挑战做出了展望.
谢阳, 宣俊. 重氮化合物作为自由基前体参与的光催化反应[J]. 有机化学, 2022, 42(12): 4247-4256.
Yang Xie, Jun Xuan. Photocatalytic Reactions Involving Diazo Compounds as Radical Precursors[J]. Chinese Journal of Organic Chemistry, 2022, 42(12): 4247-4256.
| [1] |
Gomberg, M. J. Am. Chem. Soc. 1900, 22, 757.
doi: 10.1021/ja02049a006 |
| [2] |
Yan, M.; Lo, J. C.; Edward, J. T.; Baran, P. S. J. Am. Chem. Soc. 2016, 138, 12692.
doi: 10.1021/jacs.6b08856 |
| [3] |
(a) Yi, H.; Zhang, G.-T.; Wang, H.-M.; Huang, Z.-Y.; Wang, J.; Singh, A. K.; Lei, A.-W. Chem. Rev. 2017, 117, 9016.
doi: 10.1021/acs.chemrev.6b00620 |
|
(b) Huang, C.-Y.; Li, J.-B.; Li, C.-J. Chem. Sci. 2022, 13, 5465.
doi: 10.1039/D2SC00202G |
|
|
(c) Kwon, K.; Simons, R. T.; Nandakumar, M.; Roizen, J, L. Chem. Rev. 2022, 122, 2353.
doi: 10.1021/acs.chemrev.1c00444 |
|
| [4] |
(a) Yang, Z.; Stivanin, M. L.; Jurberg, I. D.; Koenigs, R. M. Chem. Soc. Rev. 2020, 49, 6833.
doi: 10.1039/D0CS00224K |
|
(b) Durka, J.; Turkowska, J.; Gryko, D. ACS. Sustainable. Chem. Eng. 2021, 9, 8895.
doi: 10.1021/acssuschemeng.1c01976 |
|
| [5] |
Chen, Z.-L.; Empel, C.; Wang, K.; Wu, P.-P.; Cai, B.-G.; Li, L.; Koenigs, R. M.; Xuan, J. Org. Lett. 2022, 24, 2232.
doi: 10.1021/acs.orglett.2c00609 |
| [6] |
(a) Qian, L.; Cai, B.-G.; Li, L.; Xuan, J. Org. Lett. 2021, 23, 6951.
doi: 10.1021/acs.orglett.1c02555 |
|
(b) Cai, B.-G.; Li, Q.; Zhang, Q.; Li, L.; Xuan, J. Org. Chem. Front. 2021, 8, 5982.
doi: 10.1039/D1QO01102B |
|
|
(c) Zhou, S.-J.; Cai, B.-G.; Hu, C.-X.; Cheng, X.; Li, L.; Xuan, J. Chin. Chem. Lett. 2021, 32, 2577.
doi: 10.1016/j.cclet.2021.03.010 |
|
|
(d) Cai, B.-G.; Li, Q.; Li, L.; Xuan, J. Green. Synth. Catal. 2022, 3, 194.
|
|
| [7] |
(a) Lu, J.; Li, L.; He, X.-K.; Xu, G.-Y.; Xuan, J. Chin. J. Chem. 2021, 39, 1646.
doi: 10.1002/cjoc.202100064 |
|
(b) Cai, B.-G.; Luo, S.-S.; Li, L.; Li, L.; Xuan, J.; Xiao, W.-J. CCS Chem. 2020, 2, 2764.
|
|
|
(c) Cai, B.-G.; Li, L.; Xu, G.-Y.; Xiao, W.-J; Xuan, J. Photochem. Photobiol. Sci. 2021, 20, 823.
doi: 10.1007/s43630-021-00062-6 |
|
|
(d) Cheng, X.; Cai, B.-G.; Mao, H.; Lu, J.; Li, L.; Wang, K.; Xuan, J. Org. Lett. 2021, 23, 4109.
doi: 10.1021/acs.orglett.1c00979 |
|
|
(e) Ye, C.; Cai, B.-G.; Lu, J.; Cheng, X.; Li, L.; Pan, Z.-W.; Xuan, J. J. Org. Chem. 2021, 86, 1012.
doi: 10.1021/acs.joc.0c02500 |
|
| [8] |
Chen, Z.; Zheng, Y.; Ma, J.-A.; Angew. Chem., Int. Ed. 2017, 56, 4569.
doi: 10.1002/anie.201700955 |
| [9] |
Zhang, Y.; Wang, J.-H. Chem. Commun. 2009, 5390.
|
| [10] |
(a) Li, Wei.; Liu, X.-H.; Hao, X.-Y.; Hu, X.-L.; Chu, Y.-Y.; Cao, W.-D.; Qin, S.; Hu, C.-W.; Lin, L.-L.; Feng, X.-M. J. Am. Chem. Soc. 2011, 133, 15268.
doi: 10.1021/ja2056159 pmid: 21882861 |
|
(b) Li, L.; Chen, J.-J.; Li, Y.-J.; Bu, X.-B.; L, Qun.; Zhao, Y.-L. Angew. Chem., Int. Ed. 2015, 5, 12107.
pmid: 21882861 |
|
|
(c) Zhang, L.; Chen, J.-J.; Liu, S.-S.; Liang, Y.-X.; Zhao, Y.-L. Adv. Synth. Catal. 2018, 359, 351.
doi: 10.1002/adsc.201600574 pmid: 21882861 |
|
| [11] |
(a) Xuan, J.; Xiao, W.-J. Angew. Chem., Int. Ed. 2012, 51, 6828.
doi: 10.1002/anie.201200223 pmid: 32195482 |
|
(b) Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322.
doi: 10.1021/cr300503r pmid: 32195482 |
|
|
(c) Chen, Y.; Lu, L.-Q.; Yu, D.-G.; Zhu, C.-J.; Xiao, W.-J. Sci. China. Chem. 2019, 62, 24.
doi: 10.1007/s11426-018-9399-2 pmid: 32195482 |
|
|
(d) Cai, B.-G.; Xuan, J.; Xiao, W.-J.; Sci. Bull. 2019, 64, 337.
doi: 10.1016/j.scib.2019.02.002 pmid: 32195482 |
|
|
(e) Xuan, J.; He, X.-K.; Xiao, W.-J. Chem. Soc. Rev. 2020, 49, 2546.
doi: 10.1039/c9cs00523d pmid: 32195482 |
|
| [12] |
Wang, Z.-F.; Herraiz, A. G.; Hoyo, A. M. D.; Suero, M. G. Nature 2018, 554, 86.
doi: 10.1038/nature25185 |
| [13] |
Li, P.; Zhao, J.-J.; Shi, L.-J.; Wang, J.; Shi, X.-D.; Li, F.-W. Nat. Commun. 2018, 9, 1972.
doi: 10.1038/s41467-018-04331-4 |
| [14] |
Su, Y.-L.; Liu, G.-X.; Angelis, L. D.; He, R.; Al-Sayyed, A.; Schanze, K. S.; Hu, W.-H.; Qiu, H.; Doyle, M. P. ACS Catal. 2022, 12, 1357.
doi: 10.1021/acscatal.1c05611 |
| [15] |
(a) Majumdar, K. C.; Chattopadhyay, S. K. Heterocycles in Natural Product Synthesis, Wiley-VCH Verlag & Co. KGaA, Weinheim, 2011.
|
|
(b) Baumann, M.; Baxendale, I. R. J. Org. Chem. 2013, 9, 2265.
|
|
|
(c) Khanfar, M. A.; Hill, R. A.; Kaddoumi, A.; El Sayed, K. A. J. Med. Chem. 2010, 53, 8534.
doi: 10.1021/jm100941j |
|
|
(d) Rapolu, S.; Alla, M.; Bommena, V. R.; Murthy, R.; Jain, N.; Bommareddy, V. R.; Bommineni, M. R. Eur. J. Med. Chem. 2013, 66, 91.
doi: 10.1016/j.ejmech.2013.05.024 |
|
| [16] |
(a) Li, J.; Lu, X.-C.; Xu, Y.; Wen, J.-X.; Hou, G.-Q.; Liu, L. Org. Lett. 2020, 22, 9621.
doi: 10.1021/acs.orglett.0c03663 |
|
(b) Wen, J.-X.; Zhao, W.-Y.; Gao, X.; Ren, X.-F.; Dong, C.-P.; Wang, C.-L.; Liu, L.; Li, J. J. Org. Chem. 2022, 86, 1012.
doi: 10.1021/acs.joc.0c02500 |
|
| [17] |
Zhao, W.-W.; Shao, Y.-C.; Wang, A.-N.; Huang, J.-L.; He, C.-Y.; Cui, B.-D.; Wan, N.-W.; Chen, Y.-Z.; Han, W.-Y. Org. Lett. 2021, 23, 9256.
doi: 10.1021/acs.orglett.1c03603 |
| [18] |
Li, X.-D.; Golz, C.; Alcarazo, M. Angew. Chem. Int. Ed. 2021, 60, 6943.
doi: 10.1002/anie.202014775 |
| [19] |
Dong, J.-Y.; Wang, H.; Mao, S.-K.; Wang, X.; Zhou, M.-D.; Li, L. Adv. Synth. Catal. 2021, 363, 2133.
doi: 10.1002/adsc.202001436 |
| [20] |
Huang, X.-Q.; Webster, R. D.; Harms, K.; Meggers, E. J. Am. Chem. Soc. 2016, 138, 12636.
doi: 10.1021/jacs.6b07692 |
| [21] |
(a) Jurberg, I. D.; Davies, H. M. L. Chem. Sci. 2018, 9, 5112.
doi: 10.1039/c8sc01165f pmid: 29938043 |
|
(b) Zhang, Z.-Y.; Yadagiri, D.; Gevorgyan, V. Chem. Sci. 2019, 10, 8399.
doi: 10.1039/C9SC02448D pmid: 29938043 |
|
| [22] |
Fu, X.; Tang, J.; Hua, R.-Y.; Li, X.-Q.; Kang, Z.-H.; Qiu, H.; Hu, W.-H. Org. Lett. 2022, 24, 2208.
doi: 10.1021/acs.orglett.2c00516 |
| [23] |
(a) Boyd, M. R.; Hallock, Y. F.; Cardellina, J. H.; Manfredi, K. P.; Blunt, J. W.; McMahon, J. B. Buckheit, R. W.; Brignmann, G.; Schaffer, M.; Cragg, G. M.; Thomas, D. W. Johnson, G. J. J. Med. Chem. 1994, 37, 1740.
pmid: 25756503 |
|
(b) Boudesocque-Delaye, L.; Agostinho, D.; Bodet, C.; Thery- Kone, I.; Allouch, H.; Gueiffer, A.; Nuzillard, J.-M.; Enguehard- Gueiffer, C. J. Nat. Prod. 2015, 78, 597.
doi: 10.1021/np5003252 pmid: 25756503 |
|
| [24] |
Dötz, K. H. Angew. Chem. Int. Ed. 1975, 14, 644.
|
| [25] |
He, Y.-W.; Chen, H.-G.; Li, L.-Y.; Huang, J.; Xiao, T.-B.; Anand, D.; Zhou, L. J. Photochem. Photobiol. A Chem. 2018, 355, 220.
doi: 10.1016/j.jphotochem.2017.09.027 |
| [26] |
(a) Nagode, B. S.; Kant, R.; Rastogi, N. Org. Lett. 2019, 21, 6249.
doi: 10.1021/acs.orglett.9b02135 |
|
(b) Devi, L.; Pokhriyal, A.; Shekhar, S.; Kant, R.; Mukherjee, S.; Rastog, N. Asian. J. Org. Chem. 2021, 10, 3328.
doi: 10.1002/ajoc.202100518 |
|
|
(c) Li, W.-Y.; Zhou, L. Org. Lett. 2021, 23, 4279.
doi: 10.1021/acs.orglett.1c01204 |
|
| [27] |
Lednicer, D. Strategies for Organic Drug Synthesis and Design, John Wiley & Sons, Inc., Hoboken, 2008.
|
| [28] |
(a) Gibe, R.; Kerr, M. A. J. Org. Chem. 2002, 67, 6274.
|
|
(b) Li, Z.; Shi, Z.; He, C. J. Organomet. Chem. 2005, 690, 5049.
doi: 10.1016/j.jorganchem.2005.03.009 |
|
| [29] |
James, M. J.; Strieth-Kalthoff, F.; Sandfort, F.; Klauck, F.; Wagener, F.; Glorius, F. Chem.-Eur. J. 2019, 25, 8240.
doi: 10.1002/chem.201901397 |
| [30] |
Ciszewski, Ł. W.; Durka, J.; Gryko, D. Org. Lett. 2019, 21, 7028.
doi: 10.1021/acs.orglett.9b02612 pmid: 31424220 |
| [31] |
Bhattacharjee, S.; Laru, S.; Samanta, S.; Singsardar, M.; Hajra, A. RSC Adv. 2020, 10, 27984.
doi: 10.1039/d0ra05795a pmid: 35519122 |
| [32] |
(a) Dömling, A.; Wang, W.; Wang, K.; Chem. Rev. 2012, 112, 3083.
doi: 10.1021/cr100233r |
|
(b) Zhi, S.-J.; Ma, X.-M.; Zheng, W. Org. Biomol. Chem. 2019, 17, 7632.
doi: 10.1039/C9OB00772E |
|
|
(c) Lu, F.-D.; He, G.-F.; Lu, L.-Q.; Xiao, W.-J. Green. Chem. 2021, 23, 5379.
doi: 10.1039/D1GC00993A |
|
| [33] |
Xia, Y.; Qiu, D.; Wang, J.-B. Chem. Rev. 2017, 117, 13810.
doi: 10.1021/acs.chemrev.7b00382 |
| [34] |
Guo, X.; Hu, W.-H. Acc. Chem. Res. 2013, 46, 2427.
doi: 10.1021/ar300340k |
| [35] |
Su, Y.-L.; Liu, G.-X.; Liu, J.-W.; Tram, L.; Qiu, H.; Doyle, M. P. J. Am. Chem. Soc. 2020, 142, 13846.
doi: 10.1021/jacs.0c05183 |
| [36] |
Su, Y.-L.; Liu, G.-X.; Angelis, L. D.; He, R.; Al-Sayyed, A.; Schanze, K. S.; Hu, W.-H.; Qiu, H.; Doyle, M. P. ACS Catal. 2022, 12, 1357.
doi: 10.1021/acscatal.1c05611 |
| [37] |
Jiang, J.-W.; Liu, J.-J.; Yang, L.; Shao, Y.; Cheng, J.; Bao, X.-G.; Wan, X.-B.; Chem. Commun. 2015, 51, 14728.
doi: 10.1039/C5CC05183E |
| [38] |
Ma, N.; Guo, L.; Qi, D.; Gao, F.; Yang, C.; Xia, W.-J. Org. Lett. 2021, 23, 6278.
doi: 10.1021/acs.orglett.1c02071 |
| [39] |
Liu, G.-X.; Liang, H.-C.; Fu, X.; Tang, J.; Hu, W.-H.; Qiu, H. Org. Lett. 2022, 24, 4908.
doi: 10.1021/acs.orglett.2c01751 |
| [40] |
Zhang, B.; Qi, J.-Q.; Liu, Y.-H.; Li, Z.-P.; Wang, J. Org. Lett. 2022, 24, 279.
doi: 10.1021/acs.orglett.1c03941 |
| [41] |
(a) Baumgartner, T.; Réau, R. Chem. Rev. 2006, 106, 4681.
doi: 10.1021/cr040179m pmid: 17091932 |
|
(b) Baumgartner, T. Acc. Chem. Res. 2014, 47, 1613.
doi: 10.1021/ar500084b pmid: 17091932 |
|
|
(c) Joly, D.; Bouit, P.-A.; Hissler, M. J. Mater. Chem. C 2016, 4, 3686.
doi: 10.1039/C6TC00590J pmid: 17091932 |
|
| [42] |
(a) Clevenger, A. L.; Stolley, R. M.; Aderibigbe, J.; Louie, J. Chem. Rev. 2020, 120, 6124.
doi: 10.1021/acs.chemrev.9b00682 pmid: 32491839 |
|
(b) Pan, D.; Nie, G.; Jiang, S.; Li, T.; Jin, Z. Org. Chem. Front. 2020, 7, 2349.
doi: 10.1039/D0QO00473A pmid: 32491839 |
|
|
(c) Maddigan-Wyatt, J.; Hooper, J. F. Adv. Synth. Catal. 2021, 363, 924.
doi: 10.1002/adsc.202001397 pmid: 32491839 |
|
| [43] |
Jiang, H.; Jin, H.; Abdukader, A.; Lin, A.; Cheng, Y.; Zhu, C. Org. Biomol. Chem. 2013, 11, 3612.
doi: 10.1039/c3ob40429c |
| [44] |
(a) Wang, L.; Wu, Y.; Liu, Y.; Yang, H.; Liu, X.; Wang, J.; Li, X.; Jiang, J. Org. Lett. 2017, 19, 782.
doi: 10.1021/acs.orglett.6b03752 |
|
(b) Gu, X.; Xie, P.; Jiang, J.; Wu, Y.; Wang, L. J. Chem. Res. 2018, 42, 63.
doi: 10.3184/174751918X15177590137425 |
|
| [45] |
Zhou, H.-Y.; Wang, G.-G.; Wang, C.-H.; Yang, J.-Y. Org. Lett. 2022, 24, 1530.
doi: 10.1021/acs.orglett.2c00198 |
| [46] |
Kornblum, N.; DeLaMare, H. E. J. Am. Chem. Soc. 1951, 73, 880.
|
| [47] |
Li, F.; Zhu, S.-Q.; Koenigs, R. M. Chem. Commun. 2022, 58, 7526.
doi: 10.1039/D2CC02414D |
| [48] |
Wang, X.-Y.; Tong, W.-Y.; Huang, B.; Cao, S.; Li, Y.-L.; Jiao, J.-C.; Huang, H.; Yi, Q.; Qu, S.-L.; Wang, X. J. Am. Chem. Soc. 2022, 144, 4952.
doi: 10.1021/jacs.1c12874 |
| [49] |
Li, W.-Y.; Zhou, L. Org. Lett. 2022, 24, 3976.
doi: 10.1021/acs.orglett.2c01366 |
| [50] |
(a) Li, W.-Y.; Zhou, X.-Y.; Xiao, T.-B.; Ke, Z.-F.; Zhou, L. CCS Chem. 2022, 4, 638.
doi: 10.31635/ccschem.021.202000713 |
|
(b) Li, W.-Y.; Zhou, L. Green Chem. 2021, 23, 6652.
doi: 10.1039/D1GC02036F |
| [1] | 刘继宇, 李圣玉, 陈款, 朱茵, 张元. 三苯胺功能化有序介孔聚合物作为无金属光催化剂用于二硫化物合成[J]. 有机化学, 2024, 44(2): 605-612. |
| [2] | 梅青刚, 李清寒. 可见光促进C(3)(杂)芳硫基吲哚化合物的合成研究进展[J]. 有机化学, 2024, 44(2): 398-408. |
| [3] | 朱彦硕, 王红言, 舒朋华, 张克娜, 王琪琳. 烷氧自由基引发1,5-氢原子转移实现C(sp3)—H键官能团化的研究进展[J]. 有机化学, 2024, 44(1): 1-17. |
| [4] | 童红恩, 郭宏宇, 周荣. 可见光促进惰性碳-氢键对羰基的加成反应进展[J]. 有机化学, 2024, 44(1): 54-69. |
| [5] | 董江湖, 宣良明, 王池, 赵晨熙, 王海峰, 严琼姣, 汪伟, 陈芬儿. 无过渡金属或无光催化剂条件下可见光促进喹喔啉酮C(3)—H官能团化研究进展[J]. 有机化学, 2024, 44(1): 111-136. |
| [6] | 赵红琼, 于淼, 宋冬雪, 贾琦, 刘颖杰, 季宇彬, 许颖. 羧酸脱羧羟基化反应研究进展[J]. 有机化学, 2024, 44(1): 70-84. |
| [7] | 金玉坤, 任保轶, 梁福顺. 可见光介导的三氟甲基的选择性C-F键断裂及其在偕二氟类化合物合成中的应用[J]. 有机化学, 2024, 44(1): 85-110. |
| [8] | 张建涛, 张聪, 莫诺琳, 罗佳婷, 陈莲芬, 刘卫兵. 氯仿参与的烯烃自由基加成反应的研究进展[J]. 有机化学, 2023, 43(9): 3098-3106. |
| [9] | 樊思捷, 董武恒, 梁彩云, 王贵超, 袁瑶, 尹作栋, 张兆国. 可见光诱导的自由基环化反应构建4-芳基-1,2-二氢萘类化合物[J]. 有机化学, 2023, 43(9): 3277-3286. |
| [10] | 徐伟, 翟宏斌, 程斌, 汪太民. 可见光诱导的钯催化Heck反应[J]. 有机化学, 2023, 43(9): 3035-3054. |
| [11] | 冯莹珂, 王贺, 崔梦行, 孙然, 王欣, 陈阳, 李蕾. 可见光诱导的新型官能化芳基异腈化合物的二氟烷基化环化反应[J]. 有机化学, 2023, 43(8): 2913-2925. |
| [12] | 杨晓娜, 郭宏宇, 周荣. 可见光促进有机硅化合物参与的化学转化[J]. 有机化学, 2023, 43(8): 2720-2742. |
| [13] | 普佳霞, 贾小英, 韩丽荣, 李清寒. 可见光诱导C—N键断裂构建C—C键的研究进展[J]. 有机化学, 2023, 43(8): 2591-2613. |
| [14] | 王灵娜, 刘晓庆, 林钢, 金泓颖, 焦民均, 刘雪粉, 罗书平. 光促进双(4-二苯甲酮)苯醚催化C(sp3)—H键活化构建C—S键[J]. 有机化学, 2023, 43(8): 2848-2854. |
| [15] | 赵瑜, 张凯, 白育斌, 张琰图, 史时辉. 无金属条件下可见光催化与溴盐协同促进烯烃的氢硅化反应研究[J]. 有机化学, 2023, 43(8): 2837-2847. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
